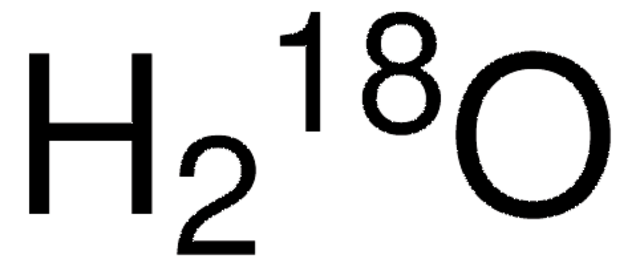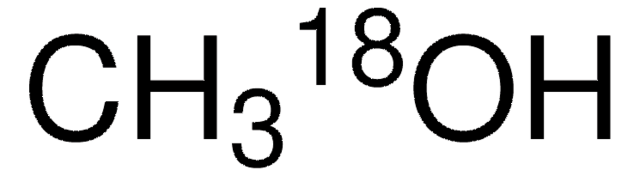About This Item
Recommended Products
description
Normalized with respect to hydrogen
isotopic purity
10 atom % 18O
form
liquid
mol wt
18.22 by atom % calculation (10 atom % 18O)
bp
100 °C (lit.)
mp
0 °C (lit.)
density
1.11 g/mL at 20 °C (lit.)
mass shift
M+2
SMILES string
[18OH2]
InChI
1S/H2O/h1H2/i1+2
InChI key
XLYOFNOQVPJJNP-NJFSPNSNSA-N
Looking for similar products? Visit Product Comparison Guide
Packaging
Storage Class Code
10 - Combustible liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
By first labeling molecular oxygen and then body water with 18O in a rat, Lifson measured the 18O abundance in expired CO2 and found the oxygens in CO2 exchanged with the oxygen in water. Indeed, these two oxygen containing species were in rapid isotopic equilibrium due to the actions of what we now know is carbonic anhydrase (Lifson, 1966)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service