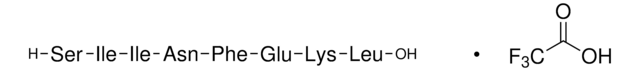Wichtige Dokumente
SRP5191
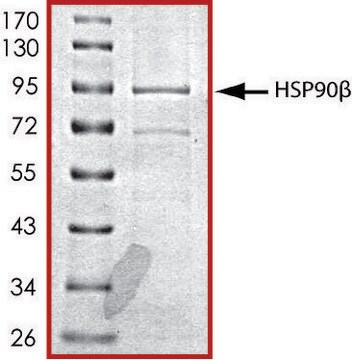
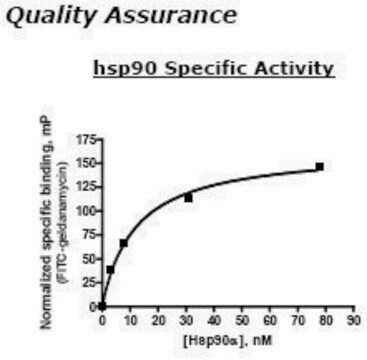
HSP90 α, His tagged human
recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution
Synonym(e):
FLJ31884, HSP86, HSP90AA1, HSP90N, HSPC1, HSPCA, HSPCAL1, HSPCAL4, HSPN, Hsp89, Hsp90, LAP2
About This Item
Empfohlene Produkte
Biologische Quelle
human
Rekombinant
expressed in baculovirus infected Sf9 cells
Assay
≥70% (SDS-PAGE)
Form
buffered aqueous glycerol solution
Mol-Gew.
~94 kDa
Methode(n)
cell culture | mammalian: suitable
Löslichkeit
water: soluble
Eignung
suitable for molecular biology
NCBI-Hinterlegungsnummer
Versandbedingung
dry ice
Lagertemp.
−70°C
Angaben zum Gen
human ... HSP90AA1(3320)
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Physikalische Form
Angaben zur Herstellung
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.