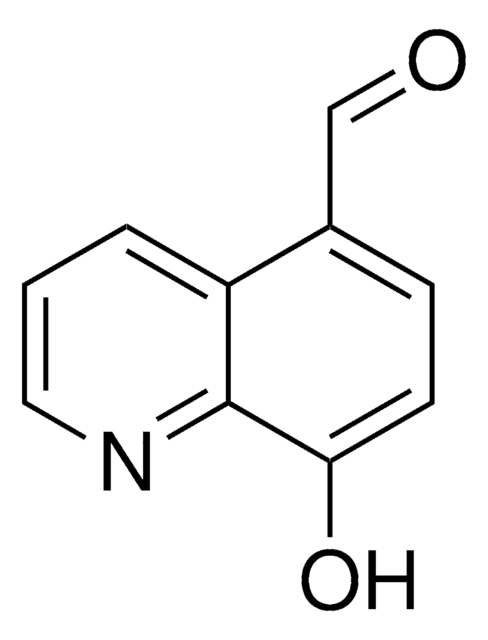
SML0067
IOX1
≥97% (HPLC)
Synonym(e):
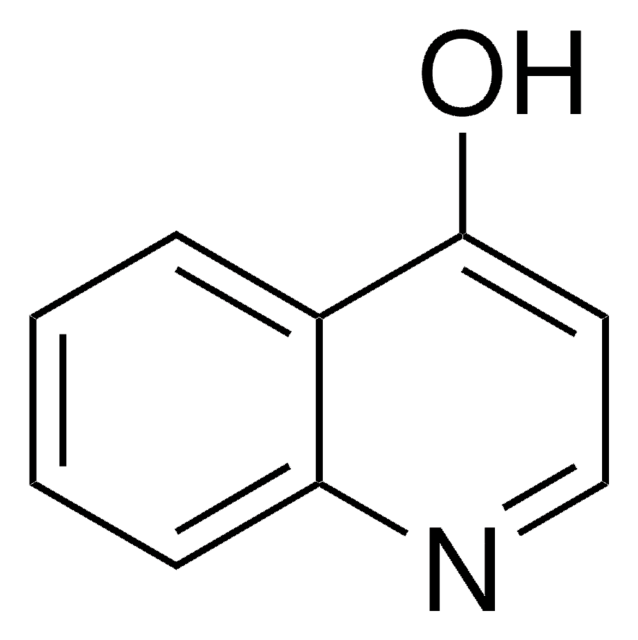
5-Carboxy-8-hydroxyquinoline, 8-Hydroxy-5-quinolinecarboxylic acid
About This Item
Empfohlene Produkte
Assay
≥97% (HPLC)
Form
powder
Farbe
white to brown
Löslichkeit
DMSO: 10 mg/mL, clear
Lagertemp.
2-8°C
SMILES String
OC(=O)c1ccc(O)c2ncccc12
InChI
1S/C10H7NO3/c12-8-4-3-7(10(13)14)6-2-1-5-11-9(6)8/h1-5,12H,(H,13,14)
InChIKey
JGRPKOGHYBAVMW-UHFFFAOYSA-N
Anwendung
- To eliminate viral latency in latent viruses in order to sensitize the viral infections to antiviral therapy.
- In histone lysine demethylase 4A (KDM4A) inhibition, to serve as a unique strategy to decrease hypoxia-inducible factors signalling.
- to restore oncogene-induced senescence in wild-type mouse embryo fibroblasts.
Biochem./physiol. Wirkung
To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
Leistungsmerkmale und Vorteile
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.