Wichtige Dokumente
P0440
Pimaricin
~2.5% (γ-irradiated Pimaricin), aqueous suspension
Synonym(e):
Natamycin
About This Item
Empfohlene Produkte
Form
aqueous suspension
Qualitätsniveau
Konzentration
~2.5% (γ-irradiated Pimaricin)
Löslichkeit
DMSO: soluble
Dichte
1.0 g/mL at 20 °C (lit.)
Wirkungsspektrum von Antibiotika
fungi
yeast
Wirkungsweise
cell membrane | interferes
Lagertemp.
2-8°C
SMILES String
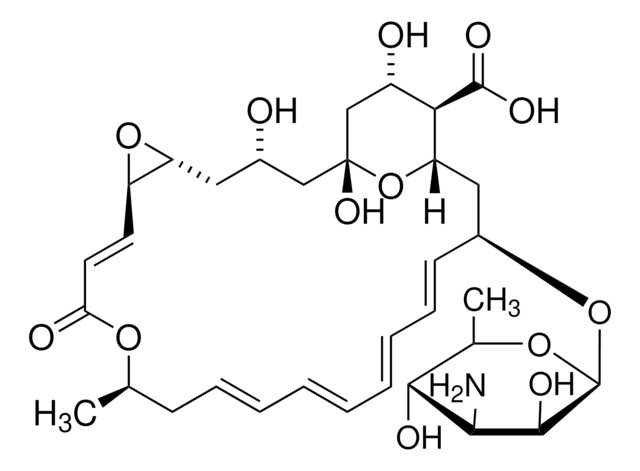
[H][C@]12C[C@@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@H](N)[C@@H]3O)\C=C\C=C\C=C\C=C\C[C@@H](C)OC(=O)\C=C\[C@@]4([H])O[C@]4([H])C[C@H](O)C[C@](O)(C[C@H](O)[C@H]1C(O)=O)O2
InChI
1S/C33H47NO13/c1-18-10-8-6-4-3-5-7-9-11-21(45-32-30(39)28(34)29(38)19(2)44-32)15-25-27(31(40)41)22(36)17-33(42,47-25)16-20(35)14-24-23(46-24)12-13-26(37)43-18/h3-9,11-13,18-25,27-30,32,35-36,38-39,42H,10,14-17,34H2,1-2H3,(H,40,41)/b4-3+,7-5+,8-6+,11-9+,13-12+/t18-,19-,20+,21+,22+,23-,24-,25+,27-,28+,29-,30+,32+,33-/m1/s1
InChIKey
NCXMLFZGDNKEPB-FFPOYIOWSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Pimaricin is a polyene antifungal antibiotic produced by Streptomyces natalensis from soil near Pietermaritzburg, South Africa.1 Pimaricin has antimicrobial activity similar to that of nystatin. In addition, it is active against Trichomonas vaginalis. Pimaricin is used in the treatment of candidiasis, trichomoniasis, fungal keratitis and aspergillosis. It has also been used as a food additive in some countries. In some studies, it has been shown to decrease the amount of mold upon which the Dermatophagoides pteronyssinus (house-dust mite) is dependent.2
Anwendung
Biochem./physiol. Wirkung
Angaben zur Herstellung
The product and any aqueous dilutions will be suspensions and should not be sterile filtered.
Lagerung und Haltbarkeit
Sonstige Hinweise
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.