Alle Fotos(1)
Wichtige Dokumente
G7134
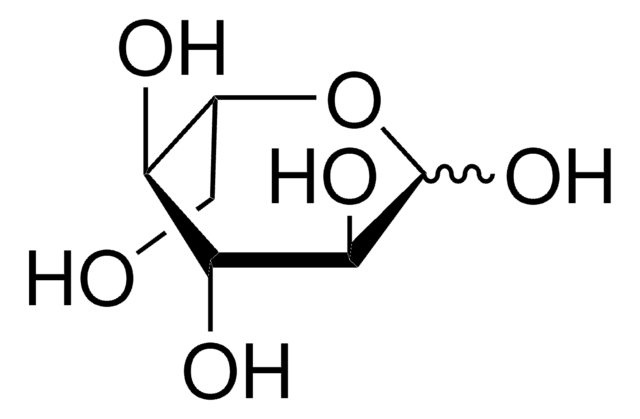
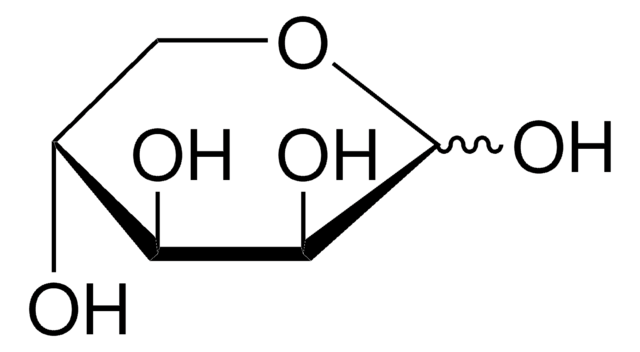
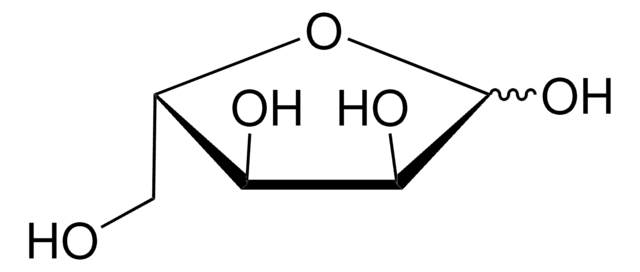
L-(−)-Galactose
≥99% (HPLC)
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H12O6
CAS-Nummer:
Molekulargewicht:
180.16
Beilstein:
1724622
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352201
PubChem Substanz-ID:
NACRES:
NA.25
Empfohlene Produkte
Qualitätsniveau
Assay
≥99% (HPLC)
Form
powder
Methode(n)
HPLC: suitable
Farbe
white
Löslichkeit
water: 50 mg/mL, clear, colorless
SMILES String
OC[C@@H]1OC(O)[C@@H](O)[C@H](O)[C@@H]1O
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3+,4+,5-,6?/m0/s1
InChIKey
WQZGKKKJIJFFOK-DHVFOXMCSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Biochem./physiol. Wirkung
L-Galactose was shown to be a key intermediate in the molecular pathway of converting D-glucose to oxalic acid in Pistia stratiotes.
Sonstige Hinweise
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Minoru Tomizawa et al.
Oncology letters, 14(1), 899-902 (2017-07-12)
Tissues surrounding hepatocellular carcinomas (HCCs) lack glucose. Hepatocyte selection medium (HSM) is deficient in glucose and is supplemented with galactose. HCC cells were cultured in HSM to investigate the stem cell markers α-fetoprotein (AFP) and cluster of differentiation 44 (CD44).
Patrick Hossler et al.
mAbs, 9(4), 715-734 (2017-04-05)
Protein glycosylation is arguably the paramount post-translational modification on recombinant glycoproteins, and highly cited in the literature for affecting the physiochemical properties and the efficacy of recombinant glycoprotein therapeutics. Glycosylation of human immunoglobulins follows a reasonably well-understood metabolic pathway, which
S E Keates et al.
Phytochemistry, 53(4), 433-440 (2000-03-24)
Axenic Pistia stratiotes L. plants were pulse-chase labeled with [14C]oxalic acid, L[1-14C]ascorbic acid, L-6-14C]ascorbic acid, D-[1-14C]erythorbic acid, L-[1-14C]galactose, or [1-14C]glycolate. Specific radioactivities of L-ascorbic acid (AsA), free oxalic acid (OxA) and calcium oxalate (CaOx) in labeled plants were compared. Samples
Mili Thakur et al.
Scientific reports, 7(1), 231-231 (2017-03-24)
Premature ovarian insufficiency (POI) is a frequent long-term complication of classic galactosemia. The majority of women with this disorder develop POI, however rare spontaneous pregnancies have been reported. Here, we evaluate the effect of D-galactose and its metabolites, galactitol and
Dimitri Daudu et al.
Frontiers in plant science, 8, 1614-1614 (2017-10-06)
Cytokinin signaling is a key regulatory pathway of many aspects in plant development and environmental stresses. Herein, we initiated the identification and functional characterization of the five CHASE-containing histidine kinases (CHK) in the economically important
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.