Alle Fotos(4)
Wichtige Dokumente
C1057
Butyrylcholinesterase aus Pferdeserum
lyophilized powder, ≥900 units/mg protein
Synonym(e):
Acylcholin-Acyl-hydrolase, Cholin-Esterase, Butyryl, Pseudocholinsterase
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(4)
About This Item
Empfohlene Produkte
Form
lyophilized powder
Qualitätsniveau
Spezifische Aktivität
≥900 units/mg protein
Mol-Gew.
tetramer 440 kDa
Zusammensetzung
Protein, ≥10%
Löslichkeit
H2O: soluble 10 mg/mL
Anwendung(en)
diagnostic assay manufacturing
Lagertemp.
−20°C
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Selective inhibition of BChE activity can be used in the detection of organophosphates. Its use in the treatment of organophosphate toxicity has been explored. It has been reported that the level of BChE in human blood correlates to the degree of protection against potentially toxic nerve agents. Cholinesterases have also been investigated for their role in Alzheimer′s disease.
Biochem./physiol. Wirkung
Butyrylcholinesterase (BChE) is a serine hydrolase that is structurally similar to acetylcholinesterase (AChE), but differs in substrate specificities and inhibitor sensitivities.BChE can, unlike AChE, efficiently hydrolyze larger esters of choline such as butyrylcholine and benzoylcholine. The enzyme is a tetrameric glycoprotein with four equal subunits (110 kDa each). The enzyme is activated by Ca2+ and Mg2+. The activity is constant over the pH range of 6.0-8.0. It is inhibited by betaine, nicotine, organophosphates, and carbamate.
Einheitendefinition
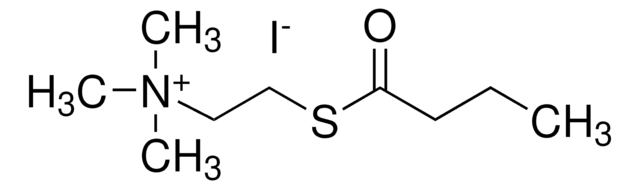
One unit will hydrolyze 1.0 μmole of butyrylcholine to choline and butyrate per min at pH 8.0 at 37 °C. The activity obtained using butyrylcholine as substrate is ~2.5 times that obtained using acetylcholine.
Physikalische Form
Highly purified; contains buffer salts
Hinweis zur Analyse
Protein determined by biuret
Inhibitor
Produkt-Nr.
Beschreibung
Preisangaben
Signalwort
Danger
H-Sätze
P-Sätze
Gefahreneinstufungen
Resp. Sens. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Tarek Mohamed et al.
Bioorganic & medicinal chemistry letters, 20(12), 3606-3609 (2010-05-18)
A group of 2,4-disubstituted pyrimidine derivatives (7a-e, 8a-e and 9a-d) that possess a variety of C-2 aliphatic five- and six-membered heterocycloalkyl ring in conjunction with a C-4 arylalkylamino substituent were designed, synthesized and evaluated as cholinesterase (ChE) inhibitors. The steric
Keriman Ozadali-Sari et al.
Bioorganic chemistry, 72, 208-214 (2017-05-10)
The present study describes the synthesis, pharmacological evaluation (BChE/AChE inhibition, Aβ antiaggregation, and neuroprotective effects), and molecular modeling studies of novel 2-[4-(4-substitutedpiperazin-1-yl)phenyl]benzimidazole derivatives. The alkyl-substituted derivatives exhibited selective inhibition on BChE with varying efficiency. Compounds 3b and 3d were found
Jianhua Liu et al.
The Journal of clinical endocrinology and metabolism, 93(5), 1980-1987 (2008-03-20)
Ghrelin, an acylated peptide hormone secreted from the gut, regulates appetite and metabolism. Elucidating its pattern of secretion in the fed and fasted states is important in the face of the obesity epidemic. Our objective was to examine changes in
R M Blong et al.
The Biochemical journal, 327 ( Pt 3), 747-757 (1998-05-15)
Butyrylcholinesterase (BChE) in human serum consists predominantly of tetramers. Recombinant BChE, however, expressed in Chinese hamster ovary (CHO) cells, consists of approx. 55% dimers, 10-30% tetramers and 15-40% monomers. To determine the origin of the monomer species we added the
Determination of butyrylcholinesterase inhibition using ion transfer across the interface between two immiscible liquids
Beattie PD, et al.
Analytical Chemistry, 66(1), 52-57 (1994)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.