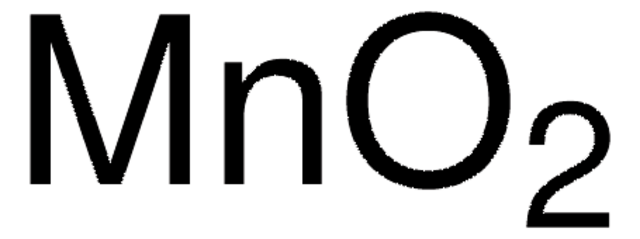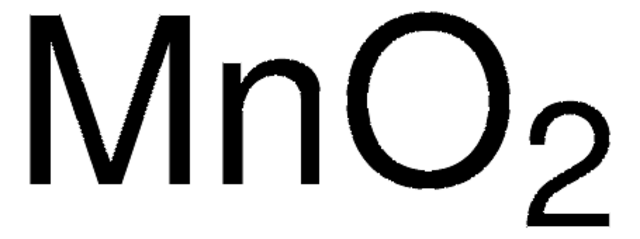Alle Fotos(2)
Wichtige Dokumente
310700
Mangan(IV)-oxid
10 μm, reagent grade, ≥90%
Synonym(e):
Mangandioxid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
MnO2
CAS-Nummer:
Molekulargewicht:
86.94
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352303
PubChem Substanz-ID:
NACRES:
NA.55
Assay:
≥90%
Qualität:
reagent grade
Form:
powder
Empfohlene Produkte
Qualität
reagent grade
Qualitätsniveau
Assay
≥90%
Form
powder
Partikelgröße
10 μm
mp (Schmelzpunkt)
535 °C (dec.) (lit.)
SMILES String
O=[Mn]=O
InChI
1S/Mn.2O
InChIKey
NUJOXMJBOLGQSY-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Manganese(IV) oxide is an oxidizing reagent that can be used for the oxidation of propargylic alcohols, benzylic or heterocyclic alcohols, saturated alcohols, 1,2-diols, allylic alcohols to α, β-ethylenic aldehydes or ketones, and amines to aldehydes, imines, amides, and diazo compounds. It can also be used for the conversion of allylic alcohols to α, β-ethylenic esters or amides, hydration of nitriles to amides, dehydrogenation and aromatization reactions.
Anwendung
- High-oxidation-state 3d metal complexes: Explores the catalytic properties of manganese(IV) oxide within high-oxidation-state complexes for advanced organic synthesis, demonstrating its critical role in accelerating chemical reactions and enhancing yield efficiencies, beneficial for pharmaceutical and chemical industries (Cheng J et al., 2018).
- Synthesis and properties of manganese complexes: Details the synthesis of new manganese complexes that demonstrate unique redox properties, useful for understanding electron transfer processes in various chemical and environmental contexts (Baffert C et al., 2002).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - STOT RE 2 Inhalation
Zielorgane
Brain
Lagerklassenschlüssel
13 - Non Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
does not flash
Flammpunkt (°C)
does not flash
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Manganese dioxide
Cahiez G, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Wen-Hui Kuan et al.
Journal of hazardous materials, 239-240, 152-159 (2012-09-25)
This study examined the reaction of methylene blue (MB) with tunneled manganese oxide pyrolusite regarding pH and reaction time. MB was cleaved through N-demethylation, in which reaction azure B (AB), azure A (AA), azure C (AC), and thionin (TH) were
Heng Lai et al.
ACS applied materials & interfaces, 4(5), 2325-2328 (2012-05-02)
MnO(2) nanoflakes coated on carbon nanohorns (CNHs) has been synthesized via a facile solution method and evaluated as anode for lithium-ion batteries. By using CNHs as buffer carrier, MnO(2)/CNH composite displays an excellent capacity of 565 mA h/g measured at
Xihong Lu et al.
Advanced materials (Deerfield Beach, Fla.), 24(7), 938-944 (2012-03-10)
WO3–x@Au@MnO2 core–shell nanowires (NWs) are synthesized on a flexible carbon fabric and show outstanding electrochemical performance in supercapacitors such as high specific capacitance, good cyclic stability, high energy density, and high power density. These results suggest that the WO3–x@Au@MnO2 NWs
Y Wang et al.
Journal of colloid and interface science, 380(1), 8-15 (2012-06-02)
Bio-inspired chemical approach has been developed for the surface modification and electrophoretic deposition of manganese dioxide and zirconia nanoparticles, prepared by chemical precipitation methods. Caffeic acid, trans-cinnamic acid, p-coumaric acid, and 2,4-dihydroxycinnamic acid were investigated for the surface modification of
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.