Wichtige Dokumente
PHR1148
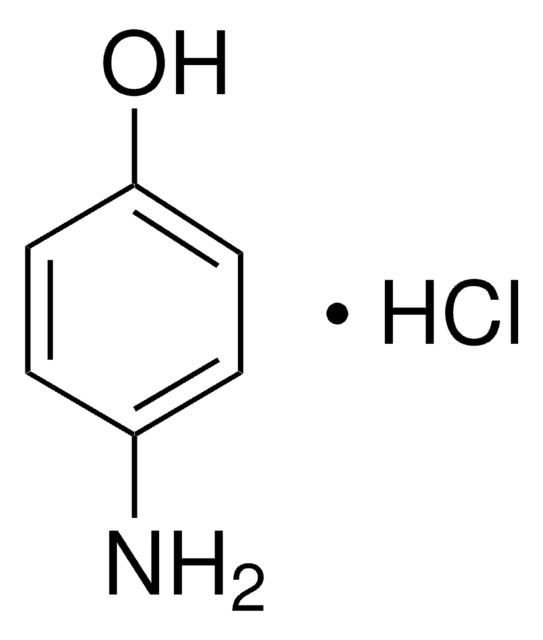
4-Aminophenol (Acetaminophen-verwandte Verbindung K) (Paracetamol-Verunreinigung K)
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(e):
4-Aminophenol, 4-Hydroxyanilin
About This Item
Empfohlene Produkte
Qualität
certified reference material
pharmaceutical secondary standard
Qualitätsniveau
Agentur
traceable to Ph. Eur. Y0001955
traceable to USP 1021204
API-Familie
mesalazine, paracetamol, acetaminophen
Analysenzertifikat (CofA)
current certificate can be downloaded
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
mp (Schmelzpunkt)
185-189 °C (lit.)
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
Lagertemp.
2-30°C
SMILES String
Nc1ccc(O)cc1
InChI
1S/C6H7NO/c7-5-1-3-6(8)4-2-5/h1-4,8H,7H2
InChIKey
PLIKAWJENQZMHA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is a primary degradation product of the widely used antipyretic and analgesic drug acetaminophen, also known as paracetamol, formed on its hydrolysis.
Anwendung
- Separation and estimation of acetaminophen and its process impurities in commercial acetaminophen tablets using high-performance liquid chromatography (HPLC)
- Simultaneous electrochemical determination of acetaminophen and its impurity K using a reduced graphene oxide-titanium nitride (RGO-TiN) nanohybrid
- Development and validation of HPLC-based stability indicating method for the determination of acetaminophen, chlorpheniramine maleate, and their possible degradation products in an over-the-counter syrup formulation
- Determination of acetaminophen, pamabrom, and their impurities using high-performance thin layer chromatography (HPTLC) and reversed phase-high performance liquid chromatography (RP-HPLC), according to the ICH guidelines, in their combined dosage tablets
- Combined analysis of acetaminophen, chlorzoxazone, and their major degradation impurities using thin-layer chromatographic (TLC)-densitometric method in their combined dosage capsules
Hinweis zur Analyse
Fußnote
Empfohlene Produkte
Ähnliches Produkt
Signalwort
Warning
Gefahreneinstufungen
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Muta. 2 - Skin Sens. 1 - STOT RE 2
Zielorgane
Kidney
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.