NIST967A
Kreatinin in gefrorenem Humanserum
NIST® SRM® 967a
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
UNSPSC-Code:
41116107
NACRES:
NA.24
Empfohlene Produkte
Qualität
certified reference material
Qualitätsniveau
Form
liquid
Verpackung
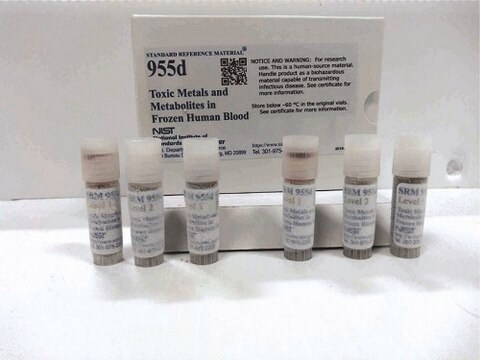
pkg of set(4) (2 ea conc)
Hersteller/Markenname
NIST®
Methode(n)
mass spectrometry (MS): suitable
Anwendung(en)
clinical testing
Format
matrix material
Allgemeine Beschreibung
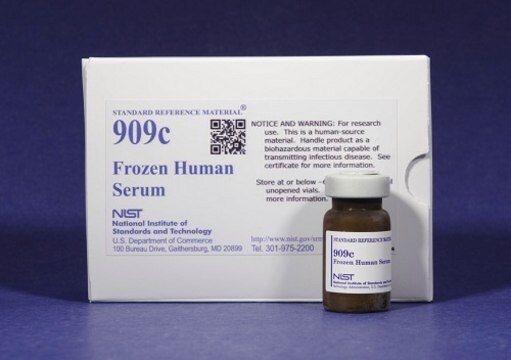
This human blood serum creatinine is a standard reference material (SRM) that consists of four stoppered vials of frozen human serum, two vials each at two different creatinine concentration levels. Each vial contains 1.0 mL of human serum.
SRM 967A_cert
SRM 967A_SDS
SRM 967A_cert
SRM 967A_SDS
Anwendung
This human blood serum creatinine is intended primarily for use in evaluating the accuracy of procedures for the determination of creatinine in human serum. It is also meant for use in validating working or secondary reference materials.
Sonstige Hinweise
- For more information on storage, usage, and expiry refer to the NIST certificate.
- Notes for biomaterials, disposal, and transport information are available in SDS.
Certified for the analytes listed below.
Creatinine
See certificate for values and more details at nist.gov/SRM.
Creatinine
See certificate for values and more details at nist.gov/SRM.
Angaben zur Herstellung
- The serum is shipped frozen on dry ice and, upon receipt, should be stored frozen until ready for use.
- Vials of the SRM to be analyzed should be removed from the freezer and allowed to stand at room temperature (20 °C to 25 °C) until thawed.
Rechtliche Hinweise
NIST is a registered trademark of National Institute of Standards and Technology
SRM is a registered trademark of National Institute of Standards and Technology
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
W Greg Miller et al.
Advances in chronic kidney disease, 25(1), 7-13 (2018-03-04)
In 2002, the Kidney Disease Outcomes Quality Initiative guidelines for identifying and treating CKD recommended that clinical laboratories report estimated glomerular filtration rate (eGFR) with every creatinine result to assist clinical practitioners to identify people with early-stage CKD. At that
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.