06054
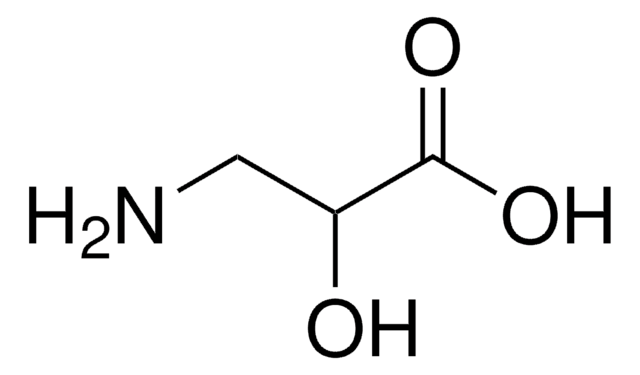
L-Isoserin
≥98.0% (TLC)
Synonym(e):
(S)-2-Hydroxy-β-alanin, (S)-3-Amino-2-hydroxy-propionsäure
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C3H7NO3
CAS-Nummer:
Molekulargewicht:
105.09
MDL-Nummer:
UNSPSC-Code:
12352209
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥98.0% (TLC)
Form
powder
Eignung der Reaktion
reaction type: solution phase peptide synthesis
Farbe
white to off-white
mp (Schmelzpunkt)
197-198 °C
Anwendung(en)
peptide synthesis
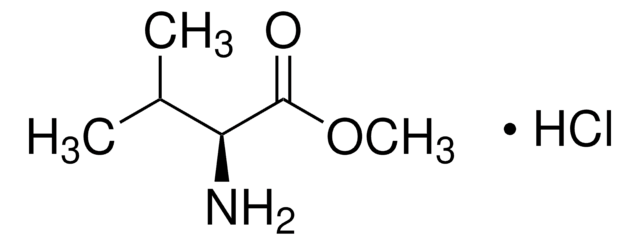
SMILES String
NC[C@H](O)C(O)=O
InChI
1S/C3H7NO3/c4-1-2(5)3(6)7/h2,5H,1,4H2,(H,6,7)/t2-/m0/s1
InChIKey
BMYNFMYTOJXKLE-REOHCLBHSA-N
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Mei-Xiang Wang et al.
The Journal of organic chemistry, 70(7), 2439-2444 (2005-03-25)
[reaction: see text] Biotransformations of a number of differently substituted and configured oxiranecarbonitriles using Rhodococcus sp. AJ270, a microbial whole-cell catalyst that contains nitrile hydratase/amidase, were studied. While almost all trans-configured 3-aryl-2-methyloxiranecarbonitriles and 2,3-dimethyl-3-phenyloxiranecarbonitrile were efficiently hydrated by the action
Microbial resolution of 2-hydroxy-3-nitropropionic acid for synthesis of optically active isoserine.
Y Yasohara et al.
Bioscience, biotechnology, and biochemistry, 65(5), 1258-1260 (2001-07-07)
The biocatalytic stereoselective hydrolysis of 2-hydroxy-3-nitropropionic acid esters was studied. Forty enzymes and three hundred microorganism strains were examined for their ability to hydrolyze ethyl 2-hydroxy-3-nitropropionic acid. Nocardia globerula IFO13150 gave n-butyl (R)-2-hydroxy-3-nitropropionate with a 92% enantiomeric excess (ee) and
Jan Cz Dobrowolski et al.
Physical chemistry chemical physics : PCCP, 12(36), 10818-10830 (2010-07-10)
The IR low-temperature Ar and Kr matrix spectra of l-isoserine were registered for the first time and interpreted by means of the anharmonic DFT frequencies calculated at the B3LYP/aug-cc-pVTZ and B3LYP/aug-cc-pVDZ levels. 54 l-isoserine conformers were predicted to be stable
Alexander Titz et al.
Bioorganic & medicinal chemistry, 18(1), 19-27 (2009-12-02)
The selectin-leukocyte interaction is the initial event in the early inflammatory cascade. This interplay proceeds via the terminal tetrasaccharide sialyl Lewis(x) (sLe(x)), present on physiological selectin ligands and E- and P-selectins located on the endothelial surface. Blocking this process is
J Du et al.
Nucleosides & nucleotides, 17(1-3), 1-13 (1998-08-26)
Asymmetric synthesis of N-substituted oxazolidinyl nucleosides has been accomplished from L-isoserine, trans- and cis-Oxazolidine intermediates (4 and 5) were stereoselectively constructed from N-protected L-isoserine with a menthoxycarbonyl group by the condensation with benzoyloxy acetaldehyde dimethyl acetal in a ratio of
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.