CC050
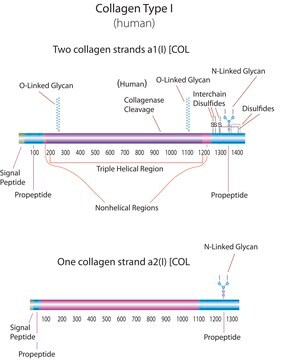
Human Collagen Type I
About This Item
Empfohlene Produkte
Biologische Quelle
human
Qualitätsniveau
Assay
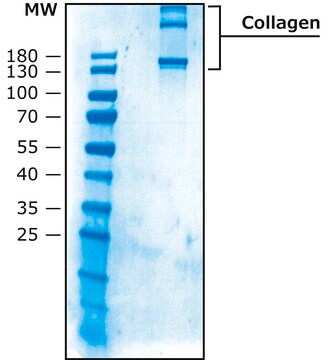
>90% (collagen type I, SDS-PAGE)
Form
liquid
Hersteller/Markenname
Chemicon®
Konzentration
1 mg/mL
Methode(n)
cell culture | mammalian: suitable
Verunreinigungen
<1% collagen type II,IV-VI & non-collagen proteins.
<10% collagen type III
Aufnahme
sample type pancreatic stem cell(s)
sample type mesenchymal stem cell(s)
sample type epithelial cells
sample type induced pluripotent stem cell(s)
sample type neural stem cell(s)
sample type: human embryonic stem cell(s)
sample type hematopoietic stem cell(s)
Löslichkeit
water: soluble at 20 °C
NCBI-Hinterlegungsnummer
UniProt-Hinterlegungsnummer
Bindungsspezifität
Peptide Source: Elastin
Peptide Source: Fibronectin
Versandbedingung
dry ice
Lagertemp.
−20°C
Angaben zum Gen
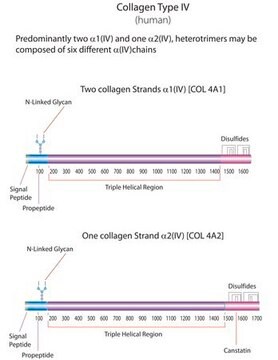
human ... COL1A1(1277)
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- as a control in the 2B4 nuclear factor of activated T-cells (NFAT)–GFP reporter cell assay, where its interaction with reporter cells can be evaluated for nuclear factor of activated T-cells (NFAT) promoter-driven GFP expression
- for coating glass slides in dynamic binding assays to create a substrate for the specific binding and study of platelets and conjugates in flow channels
- as a protein standard in histological analysis of lung tissue samples, providing a reference for the composition and characterization of extracellular matrix (ECM) components
Biochem./physiol. Wirkung
Physikalische Form
Hinweis zur Analyse
Rechtliche Hinweise
Haftungsausschluss
Lagerklassenschlüssel
12 - Non Combustible Liquids
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Extracellular matrix proteins such as laminin, collagen, and fibronectin can be used as cell attachment substrates in cell culture.
Verwandter Inhalt
This page covers the ECM coating protocols developed for four types of ECMs on Millicell®-CM inserts, Collagen Type 1, Fibronectin, Laminin, and Matrigel.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.