810226P
Avanti
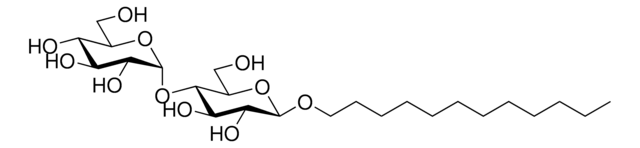
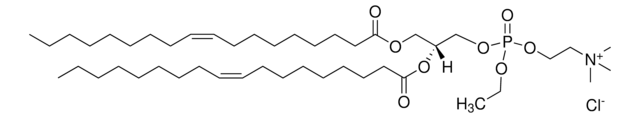
C6-NBD Lactosyl Ceramide
Avanti Polar Lipids 810226P, powder
Synonym(e):
N-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-D-lactosyl-β1-1′-sphingosine
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C42H69N5O16
CAS-Nummer:
Molekulargewicht:
900.02
UNSPSC-Code:
12352211
NACRES:
NA.25
Empfohlene Produkte
Assay
>99% (TLC)
Form
powder
Verpackung
pkg of 1 × 250 μg (810226P-250ug)
Hersteller/Markenname
Avanti Polar Lipids 810226P
Versandbedingung
dry ice
Lagertemp.
−20°C
Verwandte Kategorien
Allgemeine Beschreibung
C6-NBD Lactosyl ceramide is a commercially available fluorescent analog of compound lactosyl ceramide. Lactosyl ceramide is present on neutrophils and macrophages.
Anwendung
C6-NBD Lactosyl Ceramide has been used in:
- lactosylceramide synthase assay as a fluorescent acceptor substrate identified using fluorescence based detector in HPLC(1)
- phospholipid labeling and fluorescence-lifetime imaging microscopy (FILM) of cell membranes(6)
Biochem./physiol. Wirkung
Lactosyl ceramide is utilized for synthesis of various glycosphingolipids like oligoglycosylceramides and gangliosides in vertebrates. It is an important molecule involved in signaling cascades leading to phenotypic changes such as adhesion, migration, cell proliferation and angiogenesis.
Verpackung
5 mL Amber Glass Screw Cap Vial (810226P-250ug)
Rechtliche Hinweise
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Lagerklassenschlüssel
11 - Combustible Solids
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
W I Weis et al.
Annual review of biochemistry, 65, 441-473 (1996-01-01)
Lectins are responsible for cell surface sugar recognition in bacteria, animals, and plants. Examples include bacterial toxins; animal receptors that mediate cell-cell interactions, uptake of glycoconjugates, and pathogen neutralization; and plant toxins and mitogens. The structural basis for selective sugar
S Hakomori et al.
Journal of biochemistry, 118(6), 1091-1103 (1995-12-01)
Glycosphingolipids (GSLs), cell type-specific markers which change dramatically during ontogenesis and oncogenesis, have been implicated as playing major roles in cellular interactions and control of cell proliferation in multicellular organisms. These functional roles have been partially clarified through two types
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.