Wichtige Dokumente
T7650
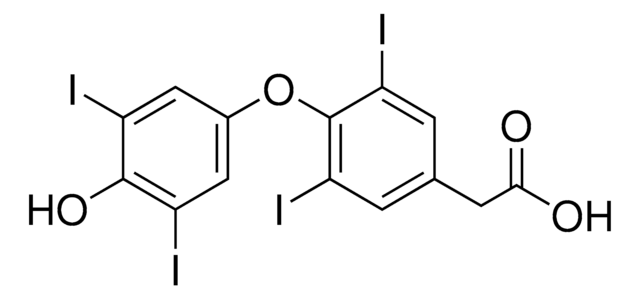
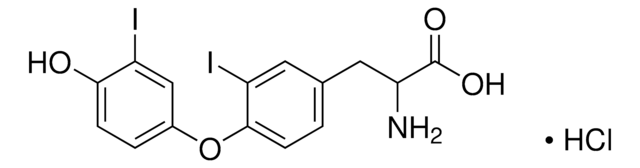
3,3′,5-Triiodothyroessigsäure
≥90%
Synonym(e):
4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenylacetic acid
About This Item
Empfohlene Produkte
Assay
≥90%
Form
powder
Lagertemp.
−20°C
SMILES String
OC(=O)Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1
InChI
1S/C14H9I3O4/c15-9-6-8(1-2-12(9)18)21-14-10(16)3-7(4-11(14)17)5-13(19)20/h1-4,6,18H,5H2,(H,19,20)
InChIKey
UOWZUVNAGUAEQC-UHFFFAOYSA-N
Angaben zum Gen
human ... THRA(7067) , THRB(7068)
rat ... Thra(81812) , Thrb(24831)
Anwendung
- Thyroid hormone research: A study explored the impact of 3,3′,5-Triiodothyroacetic acid on peripheral and neurodevelopmental findings in patients with MCT8 deficiency, highlighting its potential in therapeutic interventions for thyroid-related developmental disorders (Unsal and Hayran, 2024).
- Neurological disorder management: Research demonstrated the use of 3,3′,5-Triiodothyroacetic acid in addressing impaired T3 uptake and action in cerebral organoids modeling Allan-Herndon-Dudley syndrome, providing insights into its application in managing brain-specific thyroid hormone transport abnormalities (Salas-Lucia et al., 2024).
- Pharmaceutical development for antiviral therapies: Tiratricol, a derivative of 3,3′,5-Triiodothyroacetic acid, was identified as an inhibitor of yellow fever virus replication by targeting the viral RNA-dependent RNA polymerase, showcasing its potential in antiviral drug development (Ren et al., 2023).
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Lagerklassenschlüssel
13 - Non Combustible Solids
WGK
WGK 3
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.