P38706
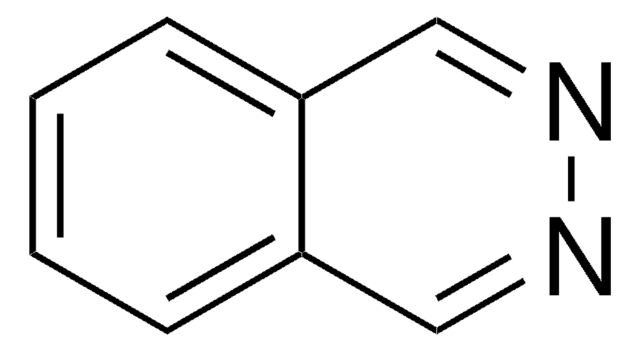
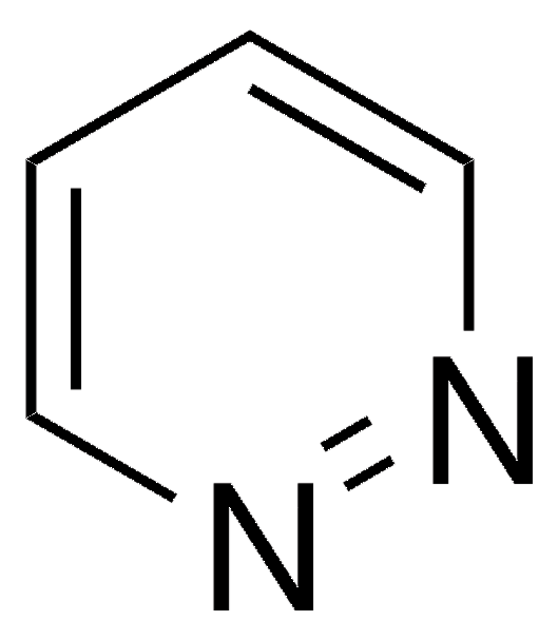
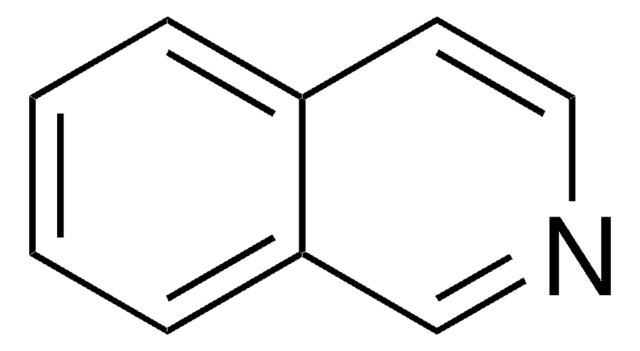
Phthalazin
98%
Synonym(e):
β-Phenodiazine, 2,3-Benzodiazine, 2,3-Diazanaphthalene, Benzo[d]pyridazine
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C8H6N2
CAS-Nummer:
Molekulargewicht:
130.15
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
98%
Form
crystals
bp
189 °C/29 mmHg (lit.)
mp (Schmelzpunkt)
89-92 °C (lit.)
Lagertemp.
2-8°C
SMILES String
c1ccc2cnncc2c1
InChI
1S/C8H6N2/c1-2-4-8-6-10-9-5-7(8)3-1/h1-6H
InChIKey
LFSXCDWNBUNEEM-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Aquatic Chronic 3 - Muta. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Mehdi Rashidi et al.
Inorganic chemistry, 49(18), 8435-8443 (2010-08-18)
The reaction of phthalazine with the binuclear organoplatinum complexes [Me(2)Pt(μ-SMe(2))(μ-dppm)PtR(2)], R = Me, Ph, 4-tolyl or R(2) = (CH(2))(4), dppm = bis(diphenylphosphino)methane, gives the corresponding complexes [Me(2)Pt(μ-phthalazine)(μ-dppm)PtR(2)] by displacement of the bridging dimethylsulfide ligand. The structures of [Me(2)Pt(μ-SMe(2))(μ-dppm)PtMe(2)] and [Me(2)Pt(μ-phthalazine)(μ-dppm)PtMe(2)]
Manuel Sánchez-Moreno et al.
The Journal of antimicrobial chemotherapy, 67(2), 387-397 (2011-12-01)
To evaluate the in vitro leishmanicidal activity of imidazole-based (1-4) and pyrazole-based (5-6) benzo[g]phthalazine derivatives against Leishmania infantum and Leishmania braziliensis. The in vitro activity of compounds 1-6 was assayed on extracellular promastigote and axenic amastigote forms, and on intracellular
Design and synthesis of selective and potent orally active S1P5 agonists.
Henri Mattes et al.
ChemMedChem, 5(10), 1693-1696 (2010-09-04)
Fadi M Awadallah et al.
European journal of medicinal chemistry, 52, 14-21 (2012-03-24)
New phthalazine-based vasodilators were synthesized through the chloroacylation of the starting compound 1-hydrazinophthalazine 4 to give the two key intermediates 5 and 7. These intermediates were used to alkylate various cyclic amines to furnish the final compounds 6a-h and 8a-h.
Michał Achmatowicz et al.
The Journal of organic chemistry, 74(2), 795-809 (2008-12-18)
p38 MAP kinase inhibitors have attracted considerable interest as potential agents for the treatment of inflammatory diseases. Herein, we describe a concise and efficient synthesis of inhibitor 1 that is based on a phthalazine scaffold. Highlights of our approach include
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.