Alle Fotos(2)
Wichtige Dokumente
D57558
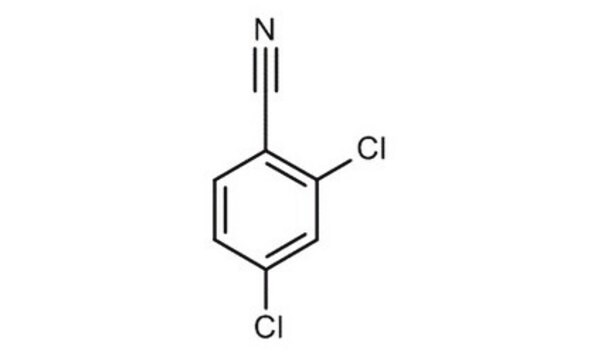
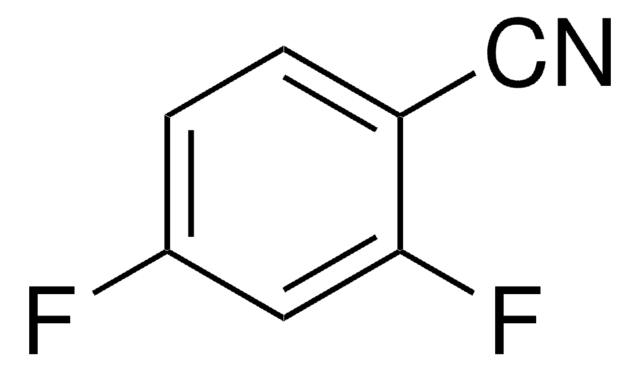
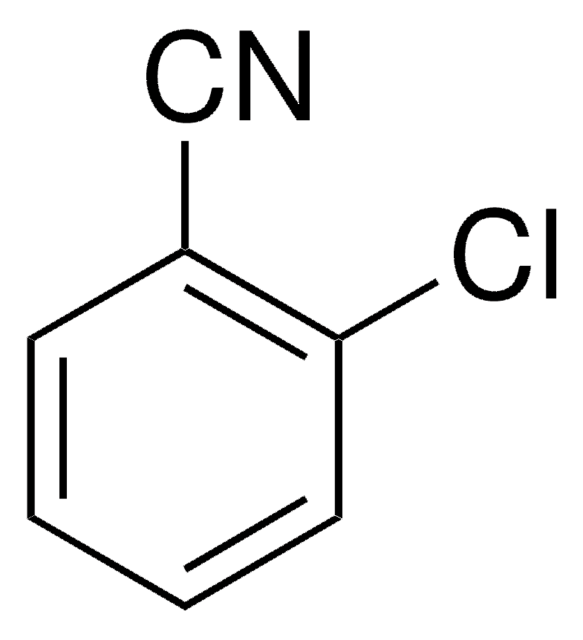
2,6-Dichlorbenzonitril
97%
Synonym(e):
Dichlobenil
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
Cl2C6H3CN
CAS-Nummer:
Molekulargewicht:
172.01
Beilstein:
1909167
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
powder
mp (Schmelzpunkt)
143-146 °C (lit.)
SMILES String
Clc1cccc(Cl)c1C#N
InChI
1S/C7H3Cl2N/c8-6-2-1-3-7(9)5(6)4-10/h1-3H
InChIKey
YOYAIZYFCNQIRF-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
2,6-Dichlorobenzonitrile can be used as a starting material to synthesize:
- 2,6-Dichlorobenzaldehyde using lithium N, N′-dimethylethylenediaminoaluminum hydride as a reducing agent.
- 5-(2,6-Dichlorophenyl)-2H-tetrazole via gold-catalyzed nucleophilic (3 + 2) cycloaddition reaction with sodium azide.
- 2,6-Dichlorobenzamide via hydrolysis using potassium tert-butoxide as a catalyst.
- Chloro-aminoindazole by reacting with hydrazine monohydrate.
- 2,6-Dichlorobenzenecarboselenoamide by treating with Woollins′ reagent.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Dermal - Aquatic Chronic 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Synthesis of primary arylselenoamides by reaction of aryl nitriles with Woollins' reagent
Hua Guoxiong, et al.
Organic Letters, 8(23), 5251-5254 (2006)
Valeria Franceschini et al.
Stem cells (Dayton, Ohio), 27(4), 825-835 (2009-04-08)
The herbicide dichlobenil selectively causes necrosis of the dorsomedial part of olfactory neuroepithelium (NE) with permanent damage to the underlying mucosa, whereas the lateral part of the olfactory region and the nasal respiratory mucosa remain undamaged. We investigated here whether
L Peng et al.
Plant biology (Stuttgart, Germany), 15(2), 405-414 (2012-07-05)
Cellulose is the major component of plant cell walls and is an important source of industrial raw material. Although cellulose biosynthesis is one of the most important biochemical processes in plant biology, the regulatory mechanisms of cellulose synthesis are still
Fang Xie et al.
Toxicology and applied pharmacology, 272(3), 598-607 (2013-08-08)
We explored the mechanisms underlying the differential effects of two olfactory toxicants, the herbicide 2,6-dichlorobenzonitrile (DCBN) and the anti-thyroid drug methimazole (MMZ), on olfactory receptor neuron (ORN) regeneration in mouse olfactory epithelium (OE). DCBN, but not MMZ, induced inflammation-like pathological
Guang-Cai Chen et al.
Journal of hazardous materials, 188(1-3), 156-163 (2011-02-18)
The effect of lead on the adsorption of diuron and dichlobenil on multiwalled carbon nanotubes (MWCNTs) was investigated to explore the possible application of MWCNTs for removal of both herbicides from contaminated water. The adsorption of diuron and dichlobenil on
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.