Wichtige Dokumente
D194255
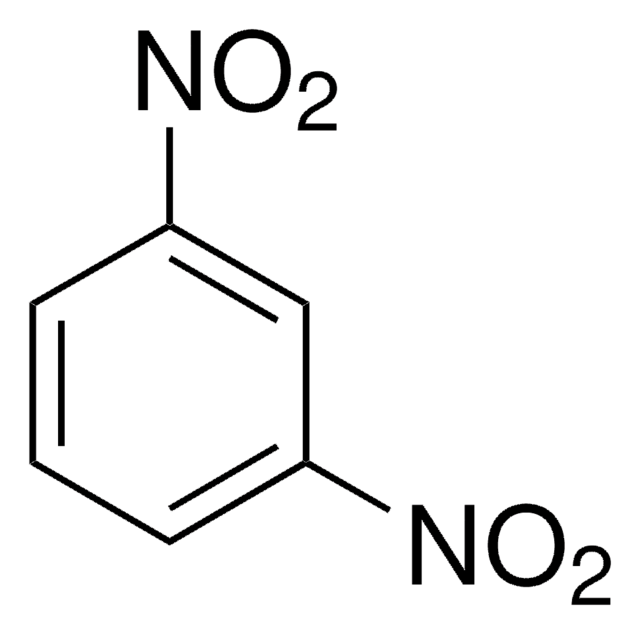
1,3-Dinitrobenzol
97% anhydrous basis
Synonym(e):
1,3-Dinitrobenzene, DNB, Meta-dinitrobenzene, m-Dinitrobenzene
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97% anhydrous basis
Form
solid
Verunreinigungen
≤10.0% H2O
bp
297 °C (lit.)
mp (Schmelzpunkt)
84-86 °C (lit.)
Dichte
1.368 g/mL at 25 °C (lit.)
SMILES String
[O-][N+](=O)c1cccc(c1)[N+]([O-])=O
InChI
1S/C6H4N2O4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4H
InChIKey
WDCYWAQPCXBPJA-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- 2′,6′- dinitrobiphenyl-4-ol and 1-nitrodibenzofuran via copper-catalyzed regioselective cross-coupling reaction with 4-iodophenol and 2-iodophenol respectively.
- 1H-indazole derivatives by reacting with various N-tosylhydrazones in the presence of a base catalyst.
- 3-(1H-Tetrazol-1-yl)benzenamine by reacting with triethyl orthoformate and NaN3 in the presence of a three-functional redox catalytic system.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Lagerklassenschlüssel
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flammpunkt (°F)
302.0 °F - closed cup
Flammpunkt (°C)
150 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| D194255-25G | 4061835150243 |
| D194255-100G | 4061833561126 |
| D194255-5G |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.