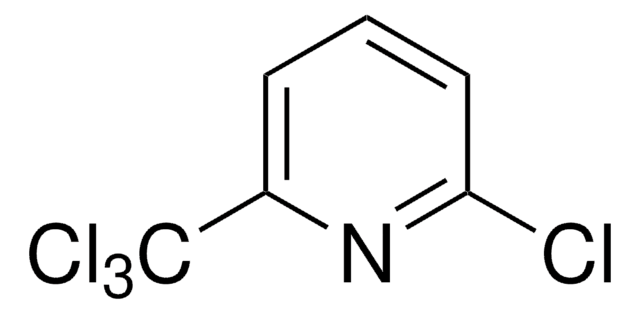
C1930
2-Chlor-6-(trichlormethyl)pyridin
≥98%
Synonym(e):
Nitrapyrin, 2-Chlor-6-trichlormethyl-pyridin, CP
About This Item
Empfohlene Produkte
Agentur
suitable for SM 5210
Qualitätsniveau
Assay
≥98%
Form
powder
Löslichkeit
ethanol: 10 mg/mL, clear, colorless to faintly yellow
SMILES String
Clc1cccc(n1)C(Cl)(Cl)Cl
InChI
1S/C6H3Cl4N/c7-5-3-1-2-4(11-5)6(8,9)10/h1-3H
InChIKey
DCUJJWWUNKIJPH-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- Biological and chemical nitrification inhibitors exhibited different effects on soil gross N nitrification rate and N(2)O production: a (15)N microcosm study.: This study examines the effects of biological and chemical nitrification inhibitors, including 2-Chloro-6-(trichloromethyl)pyridine, on soil nitrogen processes. The findings highlight the differential impacts on soil nitrogen dynamics and greenhouse gas emissions (Lan et al., 2023).
- Nitrous oxide emissions from manured soils as a function of various nitrification inhibitor rates and soil moisture contents.: This paper explores the relationship between nitrification inhibitor application rates, including 2-Chloro-6-(trichloromethyl)pyridine, and soil moisture on nitrous oxide emissions, providing insights into optimizing inhibitor use for environmental benefits (Lin and Hernandez-Ramirez, 2020).
- Nitrate losses in subsurface drainage from a corn-soybean rotation as affected by fall and spring application of nitrogen and nitrapyrin.: Investigating the seasonal application of nitrapyrin, a derivative of 2-Chloro-6-(trichloromethyl)pyridine, this study assesses its efficacy in reducing nitrate leaching and improving nitrogen use efficiency in agricultural systems (Randall and Vetsch, 2005).
- Oxidation of Nitrapyrin to 6-Chloropicolinic Acid by the Ammonia-Oxidizing Bacterium Nitrosomonas europaea.: This foundational research elucidates the microbial degradation pathway of nitrapyrin, contributing to our understanding of its environmental fate and persistence (Vannelli and Hooper, 1992).
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flammpunkt (°F)
212.0 °F - closed cup
Flammpunkt (°C)
100 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.