A47052
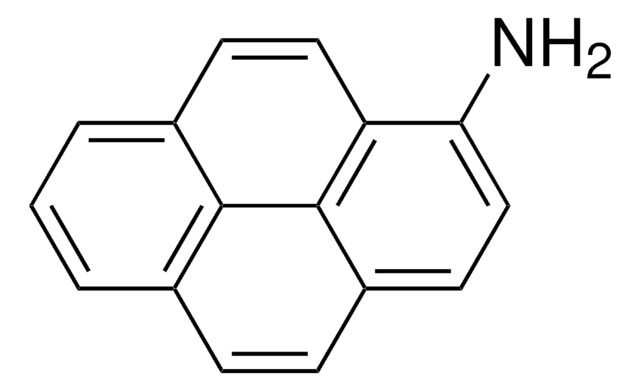
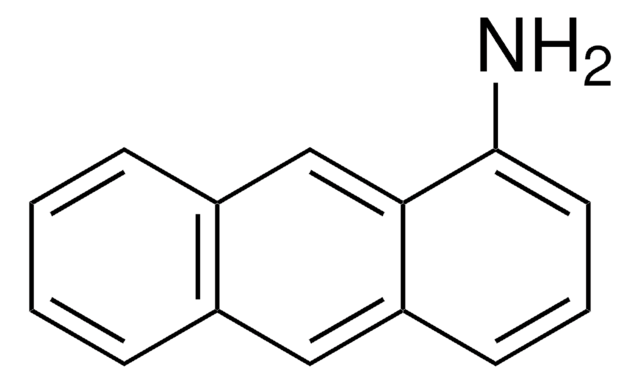
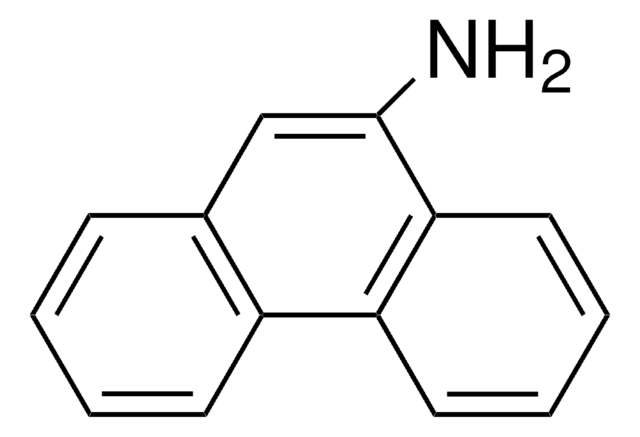
6-Aminochrysen
95%
Synonym(e):
6-Chrysenamin
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C18H13N
CAS-Nummer:
Molekulargewicht:
243.30
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
95%
Form
solid
mp (Schmelzpunkt)
209-211 °C (lit.)
SMILES String
Nc1cc2c3ccccc3ccc2c4ccccc14
InChI
1S/C18H13N/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H,19H2
InChIKey
KIVUHCNVDWYUNP-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Produces tumors in mice.
Signalwort
Warning
H-Sätze
P-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
B G Lake et al.
Toxicology and applied pharmacology, 138(2), 231-241 (1996-06-01)
Precision-cut liver slices were prepared from male Sprague-Dawley rats (pretreated with or without Aroclor 1254), male Dunkin-Hartley guinea pigs, male cynomolgus monkeys, and humans. Liver slices were cultured for 24 hr using a dynamic organ culture system in medium containing
H Yamazaki et al.
Carcinogenesis, 15(3), 465-470 (1994-03-01)
In order to address the hypothesis that 6-aminochrysene (6-AC) is converted to genotoxic products by cytochrome P450 enzymes via two activation pathways (N-hydroxylation and epoxidation), the activation of 6-AC and trans-1,2-dihydro-1,2-dihydroxy-6-aminochrysene (6-AC-diol) to genotoxic metabolites was examined in rat and
K B Delclos et al.
Carcinogenesis, 8(11), 1703-1709 (1987-11-01)
Since 6-nitrochrysene and 6-aminochrysene have shown activity in carcinogenicity bioassays, we have begun an investigation of their metabolic activation pathways and the nature of the carcinogen-DNA adducts that may be formed. N-Hydroxy-6-aminochrysene (N-hydroxy-AC), a candidate proximate or ultimate carcinogen and
K B Delclos et al.
IARC scientific publications, (124)(124), 79-86 (1993-01-01)
Carcinogenic arylamines and nitroaromatic hydrocarbons are chemicals that present occupational health hazards and share pathways of metabolic activation. The 32P-postlabelled DNA adducts formed in Chinese hamster ovary (CHO) cells treated with metabolites from two pathways that are common to the
M Mimura et al.
Drug metabolism and disposition: the biological fate of chemicals, 21(6), 1048-1056 (1993-11-01)
A cytochrome P-450 (P-450) enzyme of the CYP2B subfamily was partially purified from human liver microsomes and characterized with respect to immunochemical properties, N-terminal amino acid sequence, and catalytic activities toward typical P-450 substrates. P-450 enzymes were monitored in chromatographic
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.