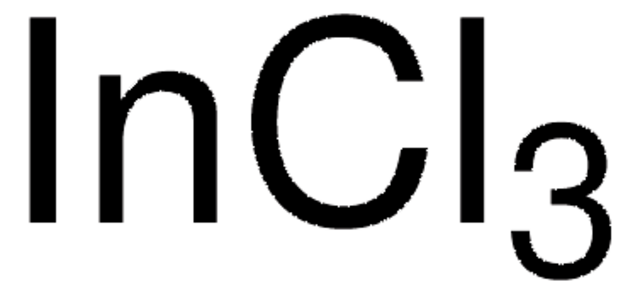Wichtige Dokumente
916013
Lithiumchlorid
anhydrous, 99.95% trace metals basis
Synonym(e):
Lithium monochloride, NSC 327172
About This Item
Empfohlene Produkte
Qualität
anhydrous
Qualitätsniveau
Assay
99.95% trace metals basis
Form
crystals
Grünere Alternativprodukt-Eigenschaften
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp (Schmelzpunkt)
605 °C (lit.)
Grünere Alternativprodukt-Kategorie
SMILES String
[Li+].[Cl-]
InChI
1S/ClH.Li/h1H;/q;+1/p-1
InChIKey
KWGKDLIKAYFUFQ-UHFFFAOYSA-M
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Click here for more information.
Anwendung
Leistungsmerkmale und Vorteile
- The anhydrous form of lithium chloride guarantees the absence of moisture, essential for preserving battery efficiency and averting undesired side reactions. Its anhydrous nature enhances reactivity, rendering it suitable for moisture-sensitive processes.
- The 99.95% purity minimizes contamination from trace metals, ensuring suitability for applications sensitive to even minute impurities.
- It contains less than 0.6% water to develop a more efficient battery component.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Lagerklassenschlüssel
13 - Non Combustible Solids
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 916013-250G | 4061842493210 |
| 916013-50G | 4061842493227 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.