525057
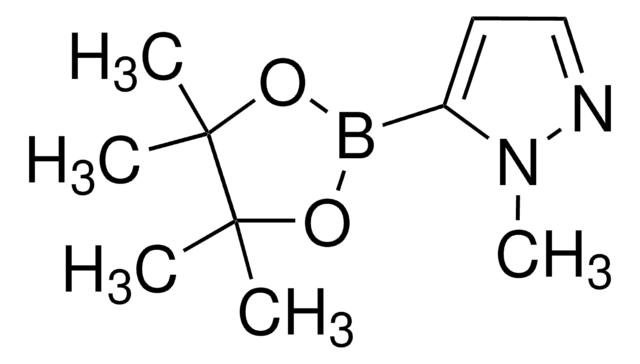
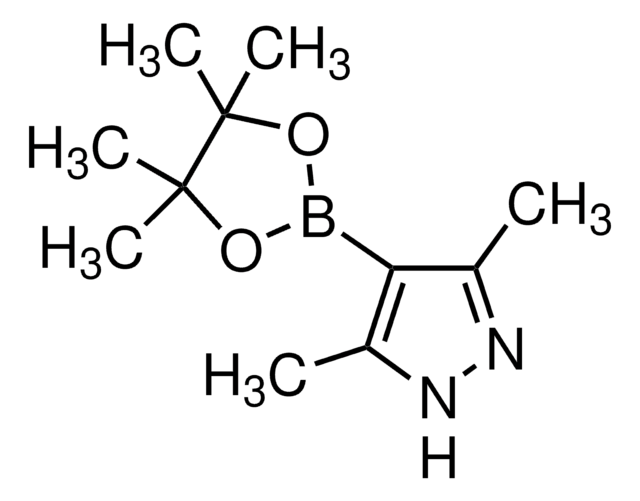
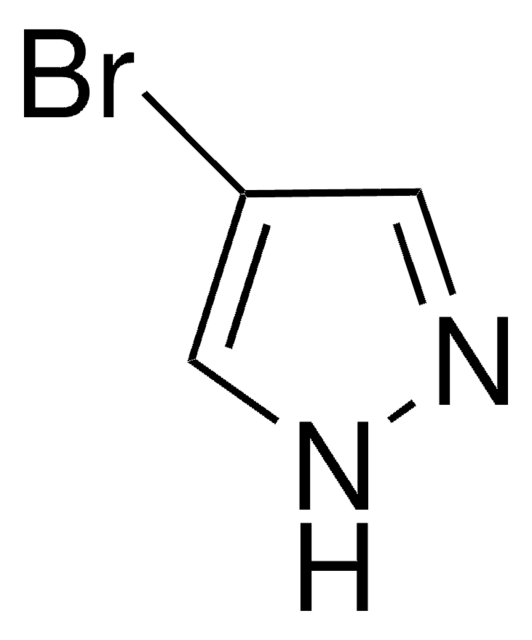
4-Pyrazolborsäurepinacolester
97%
Synonym(e):
4,4,5,5-Tetramethyl-2-(1H-pyrazol-4-yl)-1,3,2-dioxaborolan
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Lineare Formel:
(CH3)4C2O2BC3N2H3
CAS-Nummer:
Molekulargewicht:
194.04
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
solid
mp (Schmelzpunkt)
142-146 °C (lit.)
SMILES String
CC1(C)OB(OC1(C)C)c2cn[nH]c2
InChI
1S/C9H15BN2O2/c1-8(2)9(3,4)14-10(13-8)7-5-11-12-6-7/h5-6H,1-4H3,(H,11,12)
InChIKey
TVOJIBGZFYMWDT-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
Reagent used for
Reagent used in preparation of inhibitors of many highly significant therapeutic enzymes and kinases containing the privileged scaffold pyrazole, including
- Suzuki-Miyaura cross-couplings
- Ruthenium-catalyzed asymmetric hydrogenation
Reagent used in preparation of inhibitors of many highly significant therapeutic enzymes and kinases containing the privileged scaffold pyrazole, including
- VEGF
- Aurora
- Rho (ROCK)
- Janus Kinase 2 (JAK)
- c-MET
- ALK
- S-nitrosoglutathione reductase
- CDC7
- Acetyl-CoA carboxylase
- Prosurvival Bcl-2 protein
- Viral RNA-Dependent RNA polymerase
- Long Chain Fatty Acid Elongase 6
- PI3
- AKT
- Chk1
- Protein Kinase B
Rechtliche Hinweise
Product of Boron Molecular
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Mika Lindvall et al.
ACS medicinal chemistry letters, 2(10), 720-723 (2011-10-13)
A ligand-based 3D pharmacophore model for serine/threonine kinase CDC7 inhibition was created and successfully applied in the discovery of novel 2-(heteroaryl)-6,7-dihydrothieno[3,2-c]pyridin-4(5H)-ones. The pharmacophore model provided a hypothesis for lead generation missed by docking to a homology model. Medicinal chemistry exploration
Design and evaluation of 3,6-di(hetero)aryl imidazo[1,2-a]pyrazines as inhibitors of checkpoint and other kinases
Matthews, T. P.; et al.
Bioorganic & Medicinal Chemistry, 20, 4045-4049 (2010)
Asymmetric synthesis of potent chroman-based Rho kinase (ROCK-II) inhibitors
Chen, Y-T.; et al.
MedChemComm, 2, 73-75 (2011)
Michael L Curtin et al.
Bioorganic & medicinal chemistry letters, 22(9), 3208-3212 (2012-04-03)
In an effort to identify multi-targeted kinase inhibitors with a novel spectrum of kinase activity, a screen of Abbott proprietary KDR inhibitors against a broad panel of kinases was conducted and revealed a series of thienopyridine ureas with promising activity
Hassan M Shallal et al.
Bioorganic & medicinal chemistry letters, 21(5), 1325-1328 (2011-02-09)
Overexpression of prosurvival or underexpression of pro-death Bcl-2 family proteins can lead to cancer cell resistance to chemotherapy and radiation treatment. Inhibition of the prosurvival Bcl-2 family proteins has become a strategy for cancer therapy and inhibitors are currently being
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![[1,1′-Bis(diphenylphosphino)ferrocen]dichlorpalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphin)ferrocen]dichlorpalladium(II), Komplex mit Dichlormethan](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)