Alle Fotos(1)
Wichtige Dokumente
49800
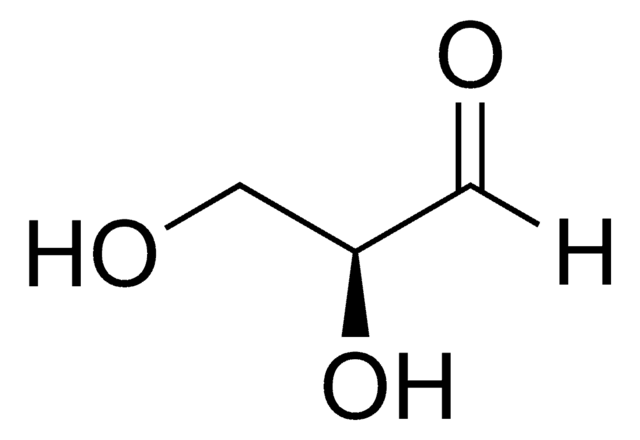
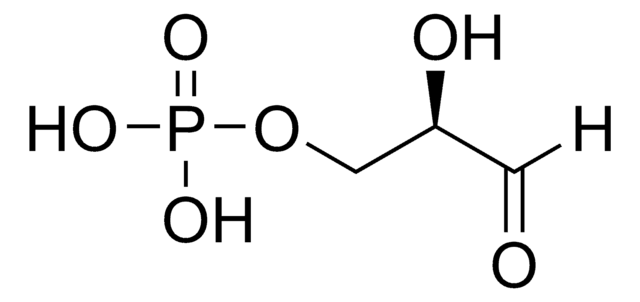
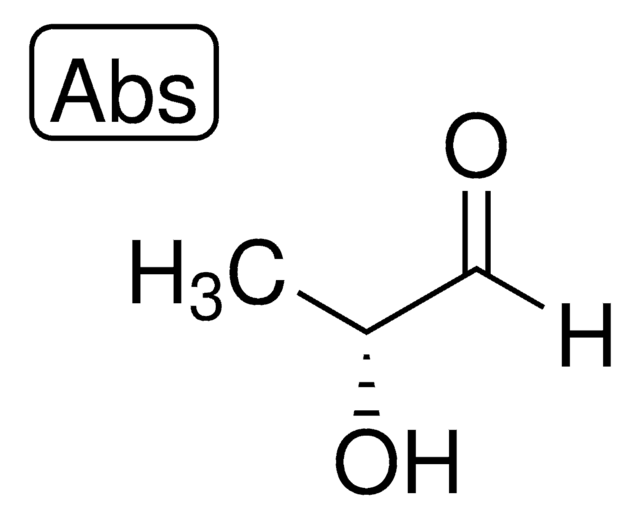
D-(+)-Glycerinaldehyd
≥98.0% (HPLC)
Synonym(e):
(2R)-2,3-Dihydroxypropanal, Triose
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C3H6O3
CAS-Nummer:
Molekulargewicht:
90.08
Beilstein:
1720474
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352200
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥98.0% (HPLC)
Verunreinigungen
≤10% water
Lagertemp.
2-8°C
SMILES String
OC[C@@H](O)C=O
InChI
1S/C3H6O3/c4-1-3(6)2-5/h1,3,5-6H,2H2/t3-/m0/s1
InChIKey
MNQZXJOMYWMBOU-VKHMYHEASA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
D-(+)-Glyceraldehyde can be utilized as a reactant in the synthesis of:
- (S)-homophenylalanine by ruthenium oxidation of a 3-amino-1,2-diol generated via coupling of an amine, and α-hydroxyaldehyde.
- β- and γ-allenols via metal-catalyzed cyclization. Allenols are used as a key precursor for the preparation of enantiopure dihydropyrans and tetrahydrooxepines.
- Isopropylidene D-glyceraldehyde intermediate, which controls the chirality in the total synthesis of prostaglandins (PGE1).
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
235.4 °F - closed cup
Flammpunkt (°C)
113 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Highly stereocontrolled one-step synthesis of anti-β-amino alcohols from organoboronic acids, amines, and α-hydroxy aldehydes
Petasis NA and Zavialov IA
Journal of the American Chemical Society, 120(45), 11798-11799 (1998)
Chiral synthesis of prostaglandins (PGE1) from D-glyceraldehyde
Stork G and Takahashi T
Journal of the American Chemical Society, 99(4), 1275-1276 (1977)
Takayoshi Wakagi et al.
PloS one, 11(1), e0147333-e0147333 (2016-01-26)
Archaea use glycolytic pathways distinct from those found in bacteria and eukaryotes, where unique enzymes catalyze each reaction step. In this study, we isolated three isozymes of glyceraldehyde oxidoreductase (GAOR1, GAOR2 and GAOR3) from the thermoacidophilic archaeon Sulfolobus tokodaii. GAOR1-3
Katarzyna Lechowicz et al.
International journal of molecular sciences, 21(16) (2020-08-13)
Lolium multiflorum/Festuca arundinacea introgression forms have been proved several times to be good models to identify key components of grass metabolism involved in the mechanisms of tolerance to water deficit. Here, for the first time, a relationship between photosynthetic and
Metal-Catalyzed Cyclization of β-and γ-Allenols Derived from d-Glyceraldehyde- Synthesis of Enantiopure Dihydropyrans and Tetrahydrooxepines: An Experimental and Theoretical Study
Alcaide BL
Chemistry?A European Journal, 15(36), 9127-9138 (2009)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.