Alle Fotos(1)
Wichtige Dokumente
431974
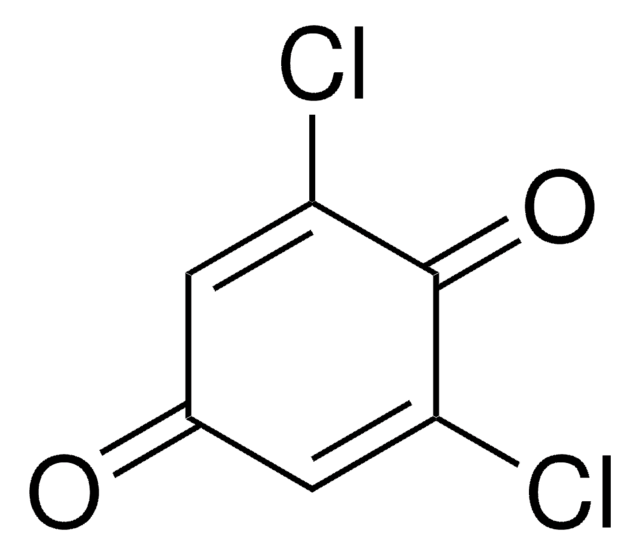
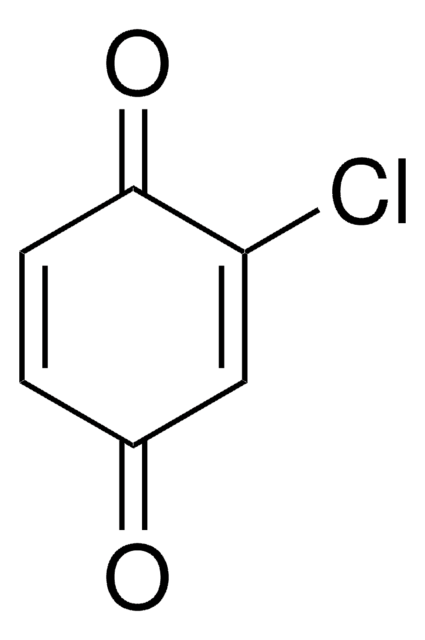
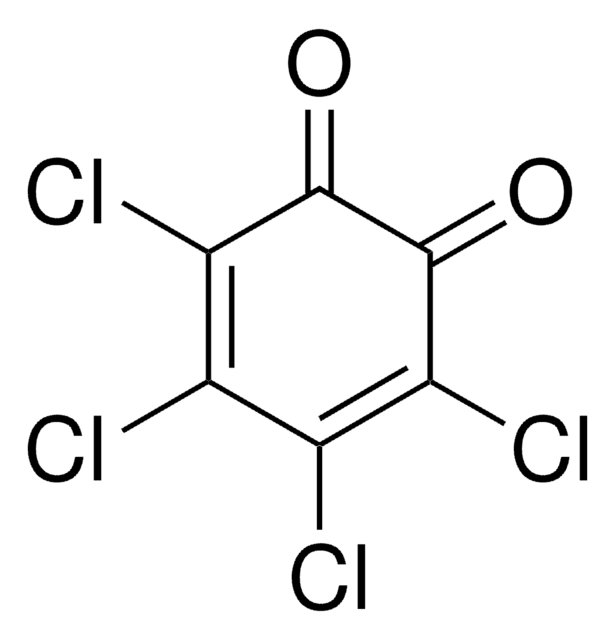
2,5-Dichlor-1,4-benzochinon
98%
Synonym(e):
2,5-Dichloro-2,5-cyclohexadiene-1,4-dione, 2,5-Dichloro-p-benzoquinone
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H2Cl2O2
CAS-Nummer:
Molekulargewicht:
176.98
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
98%
mp (Schmelzpunkt)
160-163 °C (lit.)
Funktionelle Gruppe
chloro
ketone
SMILES String
ClC1=CC(=O)C(Cl)=CC1=O
InChI
1S/C6H2Cl2O2/c7-3-1-5(9)4(8)2-6(3)10/h1-2H
InChIKey
LNXVNZRYYHFMEY-UHFFFAOYSA-N
Allgemeine Beschreibung
2,5-Dichloro-1,4-benzoquinone (DCBQ) is a halogenated quinone. DCBQs are carcinogenic intermediates.They have benn identified as chlorination disinfection byproducts in drinking water. DCBQ has been reported to increse the decomposition of a model ROOH tert-butylhydroperoxide, via formation of t-butoxyl radicals. The isomers of the DCBQ dimer have been investigated for the non-covalent interactions (NCIs) by quantum chemical calculations. Halogen bond present in 2,5-dichloro-1,4-benzoquinone have been investigated by experimental as well as theoretical charge density analysis. Its reaction with pyrrolidine has been investigated.
Anwendung
2,5-Dichloro-1,4-benzoquinone may be used in the following processes:
- As a starting material in the synthesis of asterriquinone D.
- As a model to study the utility of a novel photoreactor with LED (light-emitting diode) light source and a fibre-optic CCD (charge-coupled device) spectrophotometer.
- 2,5-dichloro-3,6-bi(3-indolyl)-1,4-hydroquinone synthesis by palladium catalyzed reaction with indole.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
B Vijaya Pandiyan et al.
Physical chemistry chemical physics : PCCP, 16(37), 19928-19940 (2014-08-15)
The competition between non-covalent interactions (NCIs), such as C-H∙∙∙O, C-H∙∙∙Cl, C-Cl∙∙∙O, C-Cl∙∙∙Cl-C, C-O∙∙∙C, C-Cl∙∙∙C and C-O∙∙∙π, in the isomers of the 2,5-dichloro-1,4-benzoquinone (DCBQ) dimer were investigated by quantum chemical calculations to study the properties of the ground and excited states.
Halogen bonding in 2, 5-Dichloro-1, 4-benzoquinone: Insights from experimental and theoretical charge density analysis.
Hathwar VR, et al.
Crystal Growth & Design, 11(5), 1855-1862 (2011)
Chun-Hua Huang et al.
Chemical research in toxicology, 28(5), 831-837 (2015-03-20)
Halogenated quinones (XQ) are a class of carcinogenic intermediates and newly identified chlorination disinfection byproducts in drinking water. Organic hydroperoxides (ROOH) can be produced both by free radical reactions and enzymatic oxidation of polyunsaturated fatty acids. ROOH have been shown
Virág Kiss et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 16(4), 519-526 (2016-12-13)
Substituted 1,4-benzoquinone (QR) derivatives are photosensitive in aqueous solution and form hydroquinones (QR-H
Construction of a photochemical reactor combining a CCD spectrophotometer and a LED radiation source.
Gombar M, et al.
Photochemical & Photobiological Sciences : Official Journal of the European Photochemistry Association and the European Society for Photobiology, 11(10), 1592-1595 (2012)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.