377945
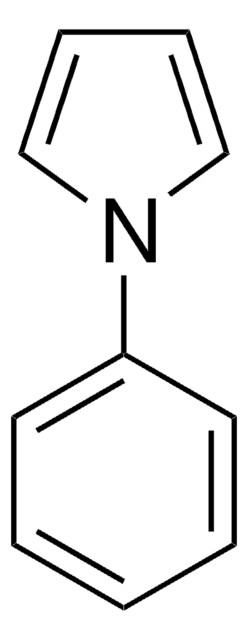
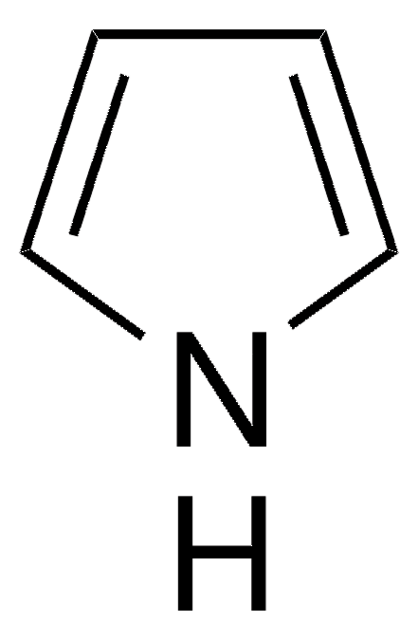
1-(Triisopropylsilyl)pyrrol
95%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C13H25NSi
CAS-Nummer:
Molekulargewicht:
223.43
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
95%
Form
liquid
Brechungsindex
n20/D 1.492 (lit.)
bp
78 °C/0.4 mmHg (lit.)
Dichte
0.904 g/mL at 25 °C (lit.)
SMILES String
CC(C)[Si](C(C)C)(C(C)C)n1cccc1
InChI
1S/C13H25NSi/c1-11(2)15(12(3)4,13(5)6)14-9-7-8-10-14/h7-13H,1-6H3
InChIKey
FBQURXLBJJNDBX-UHFFFAOYSA-N
Allgemeine Beschreibung
1-(Triisopropylsilyl)pyrrole (TISP), a heterocyclic building block, is a pyrrole derivative. TISP has been reported to generate pyrrolic cation radicals during cyclovoltammetric studies, via electroreduction. It participates in various electrophilic substitution reactions specifically at β-position, via reaction with various electrophilic reagents (Br+, I+,NO2+,etc).
Anwendung
Reagent employed in perfluoroalkylation and Vilsmeier formylation reactions.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
224.6 °F - closed cup
Flammpunkt (°C)
107 °C - closed cup
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
N-(triisopropylsilyl) pyrrole. A progenitor" par excellence" of 3-substituted pyrroles.
Bray BL, et al.
The Journal of Organic Chemistry, 55(26), 6317-6328 (1990)
Daniel A Harki et al.
Biochemistry, 41(29), 9026-9033 (2002-07-18)
Synthetic small molecules that promote viral mutagenesis represent a promising new class of antiviral therapeutics. Ribavirin is a broad-spectrum antiviral nucleoside whose antiviral mechanism against RNA viruses likely reflects the ability of this compound to introduce mutations into the viral
Observation of the cation radicals of pyrrole and of some substituted pyrroles in fast-scan cyclic voltammetry. Standard potentials and lifetimes.
Andrieux CP, et al.
Journal of the American Chemical Society, 112(6), 2439-2440 (1990)
Reaction of pyrroles with ethyl 2-nitroso-and 2-azo-propenoates, and with ethyl cyanoformate N-oxide: a comparison of the reaction pathways.
Gilchrist TL and Lemos A.
Journal of the Chemical Society. Perkin Transactions 1, 13, 1391-1395 (1993)
Synthesis, Structure, and Deoxyribonucleic Acid Sequencing with a Universal Nucleoside: 1-(2'-Deoxy-. beta.-D-ribofuranosyl)-3-nitropyrrole.
Bergstrom DE, et al.
Journal of the American Chemical Society, 117(4), Synthesis-Synthesis (1999)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.