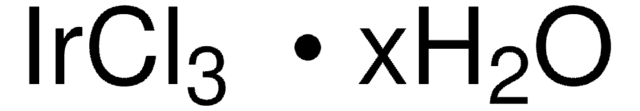Wichtige Dokumente
220930
Kaliumnitrosodisulfonat
Synonym(e):
Fremy`sches Salz
About This Item
Empfohlene Produkte
Qualität
for analytical purposes
Form
powder
Eignung der Reaktion
reagent type: oxidant
Lagertemp.
2-8°C
SMILES String
[K+].[K+].[O]N(S([O-])(=O)=O)S([O-])(=O)=O
InChI
1S/2K.H2NO7S2/c;;2-1(9(3,4)5)10(6,7)8/h;;(H,3,4,5)(H,6,7,8)/q2*+1;/p-2
InChIKey
IHSLHAZEJBXKMN-UHFFFAOYSA-L
Allgemeine Beschreibung
Anwendung
- Aromatic amines to their corresponding quinones.
- Hydroethidine to 2-hydroxyethidium.
- Tyrosine to o-quinones.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Water-react 1
Zusätzliche Gefahrenhinweise
Lagerklassenschlüssel
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
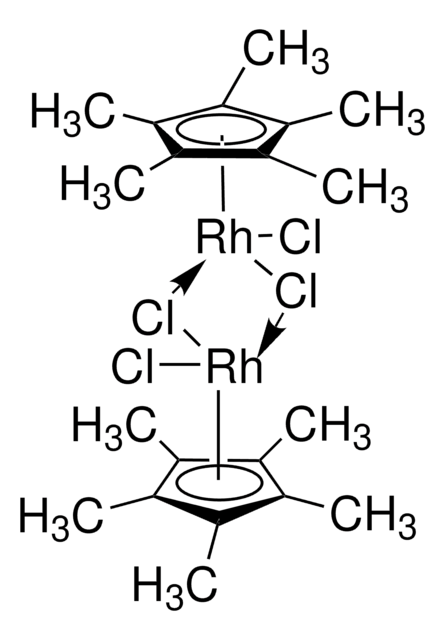
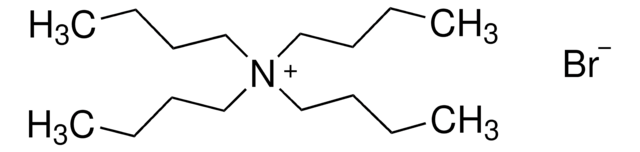
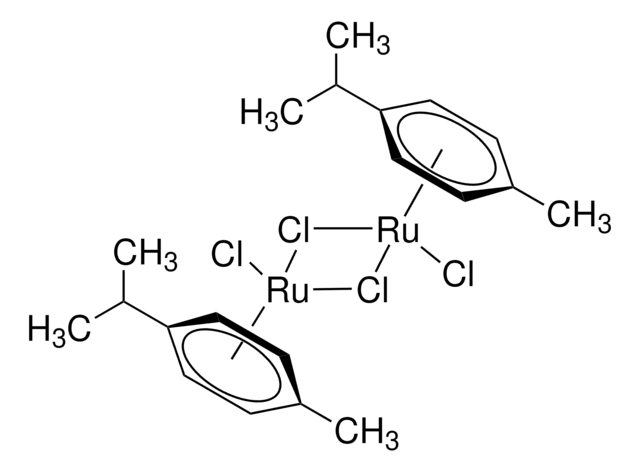
Artikel
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.