220515
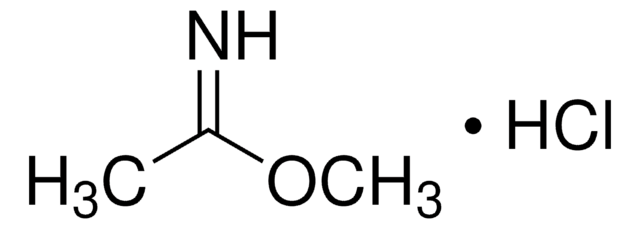
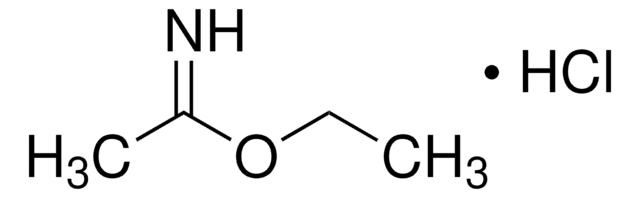
Methylbenzimidat -hydrochlorid
97%
Synonym(e):
Methyl-benzimidat -hydrochlorid, Methyl-benzolcarboximidat -hydrochlorid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
C6H5C(=NH)OCH3·HCl
CAS-Nummer:
Molekulargewicht:
171.62
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
97%
mp (Schmelzpunkt)
105-107 °C (dec.) (lit.)
Funktionelle Gruppe
ether
phenyl
Lagertemp.
−20°C
SMILES String
Cl.COC(=N)c1ccccc1
InChI
1S/C8H9NO.ClH/c1-10-8(9)7-5-3-2-4-6-7;/h2-6,9H,1H3;1H
InChIKey
HDJNHVNQRJMWSH-UHFFFAOYSA-N
Anwendung
Methyl benzimidate hydrochloride was used:
- in the synthesis of chiral phenyldihydroimidazole derivative
- as imidating reagent to modify Lys residues of cyclic Lys-Gly-Asp peptide to afford acetimidate analogs
- in the synthesis of N-benzimidoyl-(1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine)
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
R M Scarborough et al.
The Journal of biological chemistry, 268(2), 1066-1073 (1993-01-15)
Members of the snake venon-derived, "disintegrin" peptide family containing the Arg-Gly-Asp (RGD) amino acid sequence are among the most potent inhibitors of the binding of adhesive proteins to platelet glycoprotein (GP) IIb-IIIa. However, GPIIb-IIIa antagonists containing the RGD sequence are
J Einsiedel et al.
Bioorganic & medicinal chemistry letters, 11(18), 2533-2536 (2001-09-11)
Conformationally restricted benzamide bioisosteres were investigated when the chiral phenyldihydroimidazole derivative 4e (FAUC 179) showed strong and highly selective dopamine D4 receptor binding (K(i)high=0.95nM). Mitogenesis experiments indicated partial agonist properties (42%). EPC syntheses of the target compounds of type 4
Tao Ji et al.
Chemical research in toxicology, 20(4), 701-708 (2007-03-27)
Thiobenzamide (TB) is hepatotoxic in rats causing centrolobular necrosis, steatosis, cholestasis, and hyperbilirubinemia. It serves as a model compound for a number of thiocarbonyl compounds that undergo oxidative bioactivation to chemically reactive metabolites. The hepatotoxicity of TB is strongly dependent
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.