Alle Fotos(1)
Wichtige Dokumente
15149
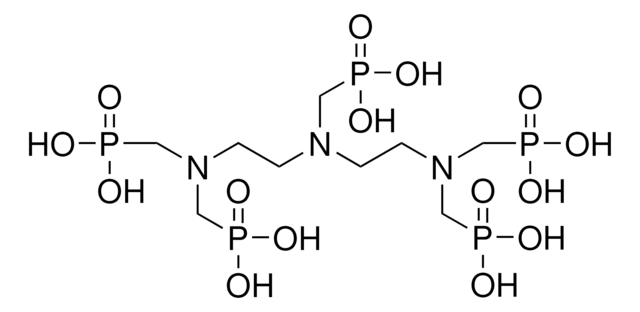
N,N-Bis-(phosphonomethyl)-glycin
≥98.0% (T)
Synonym(e):
N-(Carboxymethyl)-iminodi-(methanphosphonsäure)
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C4H11NO8P2
CAS-Nummer:
Molekulargewicht:
263.08
Beilstein:
1884944
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥98.0% (T)
Funktionelle Gruppe
amine
carboxylic acid
SMILES String
O=P(CN(CP(O)(O)=O)CC(O)=O)(O)O
InChI
1S/C4H11NO8P2/c6-4(7)1-5(2-14(8,9)10)3-15(11,12)13/h1-3H2,(H,6,7)(H2,8,9,10)(H2,11,12,13)
InChIKey
OXHDYFKENBXUEM-UHFFFAOYSA-N
Allgemeine Beschreibung
N,N-Bis(phosphonomethyl)glycine was present in the ligand bound to human apotransferrin.
Anwendung
Complexing agent; pK-values: pK1: ≤2, pK2: 2.0, pK3: 5.20, pK4: 6.77, pK5: 10.89
Biochem./physiol. Wirkung
Anti-diabetic; delay onset of diabetes in non-obese NOD mice
Signalwort
Danger
H-Sätze
P-Sätze
Gefahreneinstufungen
Eye Dam. 1
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Aaron W Michels et al.
Journal of immunology (Baltimore, Md. : 1950), 187(11), 5921-5930 (2011-11-02)
Class II major histocompatibility molecules are the primary susceptibility locus for many autoimmune disorders, including type 1 diabetes. Human DQ8 and I-A(g7), in the NOD mouse model of spontaneous autoimmune diabetes, confers diabetes risk by modulating presentation of specific islet
Markus Galanski et al.
Journal of medicinal chemistry, 46(23), 4946-4951 (2003-10-31)
A series of osteotropic (bone-seeking) [(bis(phosphonomethyl)amino-kappaN)acetato-kappaO(2-)]platinum(II) complexes attached to diammine, ethane-1,2-diamine, cis-R,S-cyclohexane-1,2-diamine, trans-S,S-cyclohexane-1,2-diamine, or trans-R,R-cyclohexane-1,2-diamine has been synthesized in accord with the concept of drug targeting and characterized by elemental analysis, (1)H, (13)C, and (31)P NMR spectroscopy. The in vitro
C T Bailey et al.
Biochemistry, 36(33), 10105-10108 (1997-08-19)
The mechanism by which the iron-transport protein transferrin releases its iron in vivo is presently unclear. In vitro studies have implicated two concurrent chelator-mediated iron-release pathways: one which is hyperbolic in nature, involving a conformational change in the protein as
W R Harris et al.
Biochemistry, 30(28), 6930-6936 (1991-07-16)
Difference ultraviolet spectroscopy has been used to monitor the binding of a series of phosphonate ligands to human apotransferrin. The ligands consist of pyrophosphate as well as the phosphonic acids (aminomethyl)phosphonic acid (AMPA), (hydroxymethyl)phosphonic acid (HMP), (phosphonomethyl)-iminodiacetic acid (PIDA), N,N-bis(phosphonomethyl)glycine
L J Olson et al.
Toxicology, 30(2), 103-114 (1984-03-01)
Exposure to the plant growth regulators, chlorocholine chloride (CCC) and glyphosine (GPS), resulted in significant immunomodulatory effects after feeding to deer mice (Peromyscus maniculatus) for 28 days. Cyclophosphamide (CP) and saline controls were included. Both CCC and GPS feeding levels
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.