117757
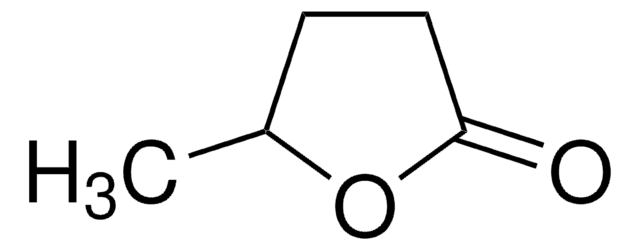
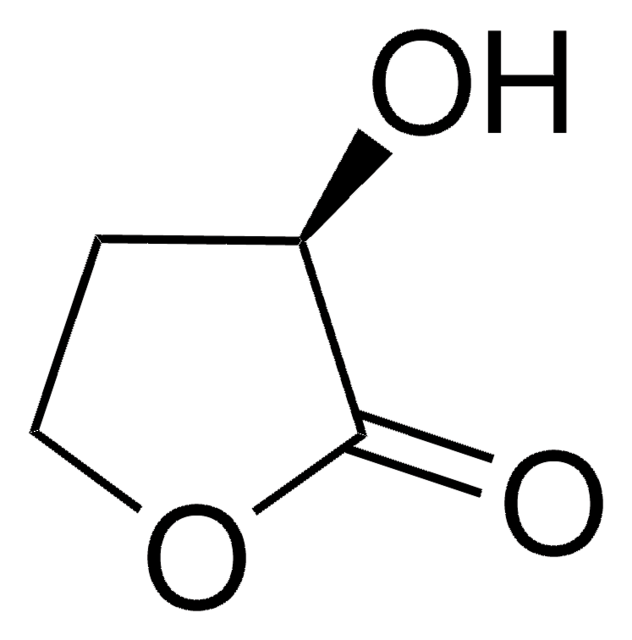
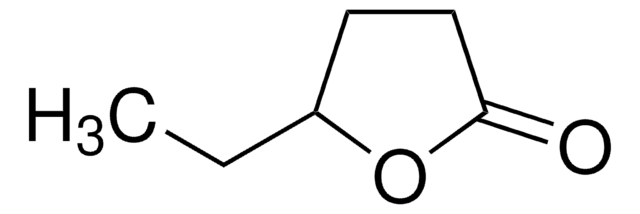
α-Methyl-γ-butyrolacton
98%
Synonym(e):
4,5-Dihydro-3-methyl-2(3H)-furanon
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C5H8O2
CAS-Nummer:
Molekulargewicht:
100.12
Beilstein:
80418
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Brechungsindex
n20/D 1.432 (lit.)
bp
78-81 °C/10 mmHg (lit.)
Löslichkeit
THF: soluble
Funktionelle Gruppe
ester
SMILES String
CC1CCOC1=O
InChI
1S/C5H8O2/c1-4-2-3-7-5(4)6/h4H,2-3H2,1H3
InChIKey
QGLBZNZGBLRJGS-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
α-Methyl-γ-butyrolactone undergoes benzylation to give racemic α-benzyl-α-methyl-γ-butyrolactone.
Anwendung
α-Methyl-γ-butyrolactone was used as model compound in Bracketing experiments to investigate the thermodynamically favored site of reaction of pilocarpine.
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
163.4 °F - closed cup
Flammpunkt (°C)
73 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
M Satterfield et al.
Journal of the American Society for Mass Spectrometry, 10(3), 209-216 (1999-03-09)
Analysis of the sites of reaction of a biologically important compound, pilocarpine, a molecule with imidazole and butyrolactone rings connected by a methylene bridge, has been accomplished in a quadrupole ion trap with the aim of characterizing its structure/reactivity relationships.
Eric B Gonzales et al.
The Journal of pharmacology and experimental therapeutics, 309(2), 677-683 (2004-01-27)
Alkyl-substituted butyrolactones have both inhibitory and stimulatory effects on GABA(A) receptors. Lactones with small alkyl substitutions at the alpha-position positively modulate the channel, whereas beta-substituted lactones tend to inhibit the GABA(A) receptor. These compounds mediate inhibition through the picrotoxin site
Hagai Tavori et al.
Bioorganic & medicinal chemistry, 16(15), 7504-7509 (2008-06-24)
Paraoxonase1 (PON1) is a HDL bound enzyme and many of the anti-atherogenic properties of HDL are attributed to PON1. The enzyme precise mechanism of protective action and its endogenous substrate remain elusive. PON1 hydrolyzes organophosphates, arylesters and lactones, whereas the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.