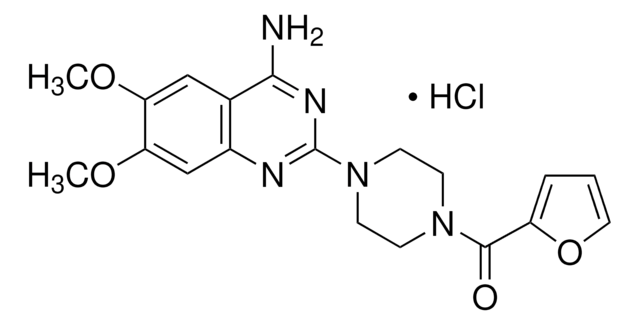
H1512
Haloperidol
≥98% (TLC), powder, dopamine receptor antagonist
Sinónimos:
4-[4-(4-Chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone, 4-[4-(4-Chlorophenyl)-4-hydroxypiperidino]-4′-fluorobutyrophenone, 4-[4-(p-Chlorophenyl)-4-hydroxypiperidino]-4′-fluorobutyrophenone
About This Item
Productos recomendados
Nombre del producto
Haloperidol, powder
form
powder
color
white
solubility
45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 0.39 mg/mL
0.1 M HCl: 3 mg/mL
DMSO: soluble
H2O: insoluble
ethanol: soluble
originator
Johnson & Johnson
SMILES string
OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c3ccc(Cl)cc3
InChI
1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2
InChI key
LNEPOXFFQSENCJ-UHFFFAOYSA-N
Gene Information
human ... ABCB1(5243) , ADRA1A(148) , ADRA2A(150) , ADRA2C(152) , CHRM1(1128) , DRD2(1813) , DRD3(1814) , DRD4(1815) , EBP(10682) , HRH1(3269) , HTR2A(3356) , HTR2C(3358) , HTR7(3363) , KCNH1(3756) , KCNH2(3757) , PRNP(5621)
rat ... Adra1a(29412) , Adra2a(25083) , Chrm1(25229) , Chrm2(81645) , Drd1a(24316) , Drd2(24318) , Drd3(29238) , Drd4(25432) , Hrh1(24448) , Htr1a(24473) , Htr1b(25075) , Htr2a(29595) , Htr2c(25187) , Oprs1(29336) , Slc6a3(24898) , Slc6a4(25553)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- in ethanol to serves as an inhibitor of Erg2p
- to address the mechanism of haloperidol in ferroptosis using hepatocellular carcinoma cells: Hep G2 and Huh-7 cell lines
- in receptor internalization assay
- as an antipsychotic drug in Dulbecco′s Modified Eagle medium
Biochem/physiol Actions
Features and Benefits
signalword
Danger
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico