62118
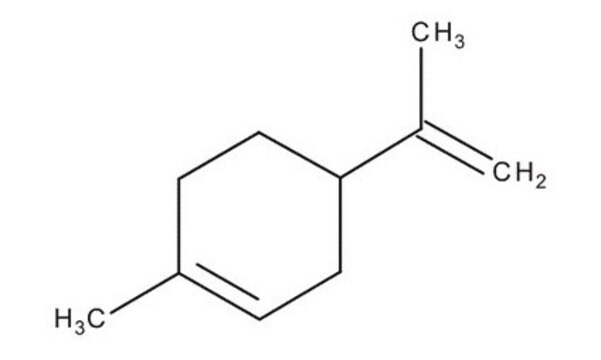
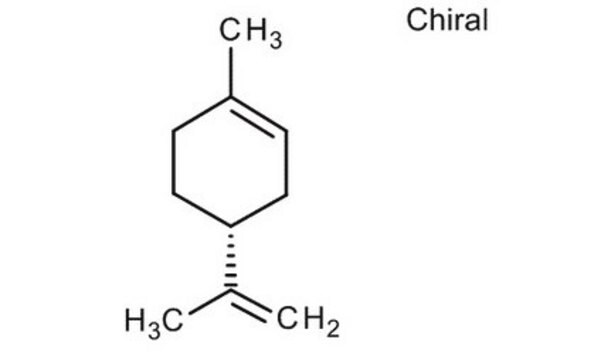
(R)-(+)-Limonene
analytical standard
Sinónimos:
(+)-p-Mentha-1,8-diene, (+)-Carvene, (R)-4-Isopropenyl-1-methyl-1-cyclohexene
About This Item
Productos recomendados
grade
analytical standard
Quality Level
vapor density
4.7 (vs air)
vapor pressure
<3 mmHg ( 14.4 °C)
assay
≥99.0% (sum of enantiomers, GC)
optical activity
[α]20/D +115.5±1°, c = 10% in ethanol
shelf life
limited shelf life, expiry date on the label
expl. lim.
6.1 %
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.473 (lit.)
n20/D 1.473
bp
176-177 °C (lit.)
density
0.842 g/mL at 20 °C (lit.)
application(s)
agriculture
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
format
neat
storage temp.
2-8°C
SMILES string
CC(=C)[C@@H]1CCC(C)=CC1
InChI
1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4,10H,1,5-7H2,2-3H3/t10-/m0/s1
InChI key
XMGQYMWWDOXHJM-JTQLQIEISA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Application
- Analysis of alcoholic beverages for the determination of (R)-(+)-limonene by differential pulse voltammetry (DPV) using two novel electrochemical sensors
- Simultaneous determination of limonene and linalool in 10 perfume product samples by two-dimensional high-performance liquid chromatographic (HPLC) method combined with electrospray ionization tandem mass spectrometry (ESI-MS/MS)
- Determination of the enantiomeric composition of volatile chiral compounds, commonly present in three plant species from the Citrus genus by multidimensional gas chromatography (MDGC) coupled to mass spectrometry (MS)
- Multi-residue analysis of volatiles and fatty acids found in wild and cultivated fennel samples by a single extraction method and gas chromatographic-flame ionization detection (GC-FID)
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
123.8 °F - closed cup
flash_point_c
51 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
-(+)-Limonene, purum, ≥98.0% (sum of enantiomers, GC); Geranyl tiglate; α-Terpineol, natural, ≥96%, FCC, FG; Geranyl formate; α-Pinene
-3,7-Dimethyl-2,6-octadien-1-ol; Neral; Geraniol; Geranial; Undecanal; Citronellyl acetate; Neryl acetate; 3,7-Dimethyl-2,6-octadienyl acetate; 1-Tetradecene; Tetradecane; α-Bisabolol
GC Analysis of Sweet Orange Essential Oil on SLB®-5ms (10 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
-Cymene; 2,5-Dimethylpyrrole; Acetoin, ≥96%, FCC, FG; 2,5-Dimethylpyrazine; 2,6-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Dimethylpyrazine; 4-Heptanone; 3-Ethylpyridine; 2,3,5-Trimethylpyrazine; Furfural; Pyrrole; Furfuryl acetate; Linalool; Linalyl acetate; 5-Methylfurfural; γ-Butyrolactone; 2-Acetyl-1-methylpyrrole; Furfuryl alcohol; 2-Acetylpyrrole; Pyrrole-2-carboxaldehyde
Contenido relacionado
Gas chromatography separates volatile compounds in the gas phase, applied in various industries for quality control.
Gas chromatography is a common analytic technique used to separate and analyze volatile compounds in the gas phase. GC is applied in many industries for quality control, and to identify and/or quantify compounds in a mixture.
Chromatograms
suitable for GCNuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico