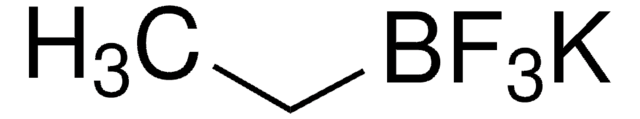
655228
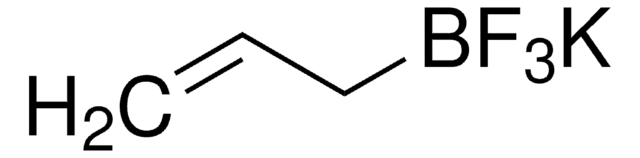
Potassium vinyltrifluoroborate
95%
Sinónimos:
Potassium (ethenyl)trifluoroborate
About This Item
Productos recomendados
Quality Level
assay
95%
form
solid
SMILES string
[K+].F[B-](F)(F)C=C
InChI
1S/C2H3BF3.K/c1-2-3(4,5)6;/h2H,1H2;/q-1;+1
InChI key
ZCUMGICZWDOJEM-UHFFFAOYSA-N
Categorías relacionadas
General description
Potassium vinyltrifluoroborate is an air- and water-stable potassium organotrifluoroborate that can be utilized in coupling reactions under relatively mild conditions.
Application
- Suzuki Miyaura cross-coupling reactions and polymerization reactions
- Synthesis of photonic crystals
- Synthesis of sensitizers for dye-sensitized solar cells
- Mannich / diastereoselective hydroamination reaction sequence
Organotrifluoroborates as versatile and stable boronic acid surrogates.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Potassium trifluoroborates are a special class of organoboron reagents that offer several advantages over the corresponding boronic acids and esters in that they are moisture- and air-stable, and are remarkably compliant with strong oxidative conditions.
These bench stable Potassium Organotrifluoroborates are useful for Suzuki-Miyaura cross-coupling reactions and have also been used for a variety of other C-C bond forming reactions. Importantly, these reagents are compatible with a wide range of functional groups and are stable to many commonly used and harsh reaction conditions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)