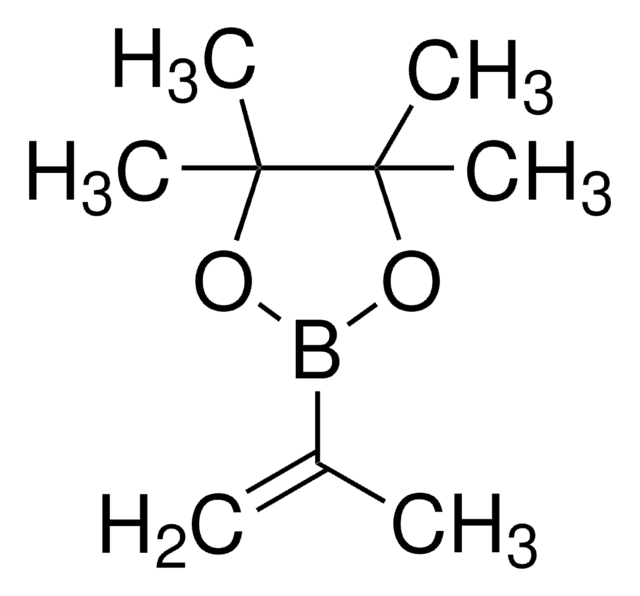
633348
Vinylboronic acid pinacol ester
contains phenothiazine as stabilizer, 95%
Sinónimos:
2-Ethenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 2-Vinyl-4,4,5,5-tetramethyl-1,3,2-dioxaoborolane, 4,4,5,5-Tetramethyl-2-vinyl-1,3,2-dioxaborolane
About This Item
Productos recomendados
Quality Level
assay
95%
contains
phenothiazine as stabilizer
refractive index
n20/D 1.4300 (lit.)
density
0.908 g/mL at 25 °C (lit.)
storage temp.
−20°C
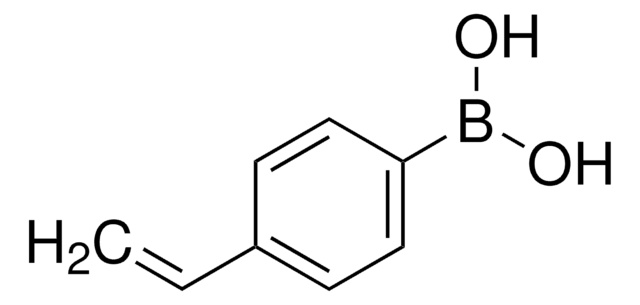
SMILES string
CC1(C)OB(OC1(C)C)C=C
InChI
1S/C8H15BO2/c1-6-9-10-7(2,3)8(4,5)11-9/h6H,1H2,2-5H3
InChI key
DPGSPRJLAZGUBQ-UHFFFAOYSA-N
Application
- Suzuki-Miyaura coupling reactions
- Mizoroki-Heck reactions (cascade reaction)
- Intramolecular Nozaki-Hiyama-Kishi reactions
- Stereoselective Cu-catalyzed γ-selective and stereospecific coupling
- Control of stereoselectivity and mechanistic portrait on intramolecular (4+1)-cycloaddition of dialkoxycarbenes
- Regio- and stereoselective synthesis of trisubstituted alkenes via gold(I)-catalyzed hydrophosphoryloxylation of haloalkynes followed by Pd-catalyzed consecutive cross-coupling reactions
- Asymmetric Birch reductive alkylation
Reagent used in Preparation of
- Molecular tubes for lipid sensing
- Enzymatic inhibitors, antibiotics, receptor analogs, and other biologically significant compounds (including total syntheses)
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 2 - Flam. Liq. 3 - Skin Sens. 1
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
93.2 °F
flash_point_c
34 °C
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)