43153
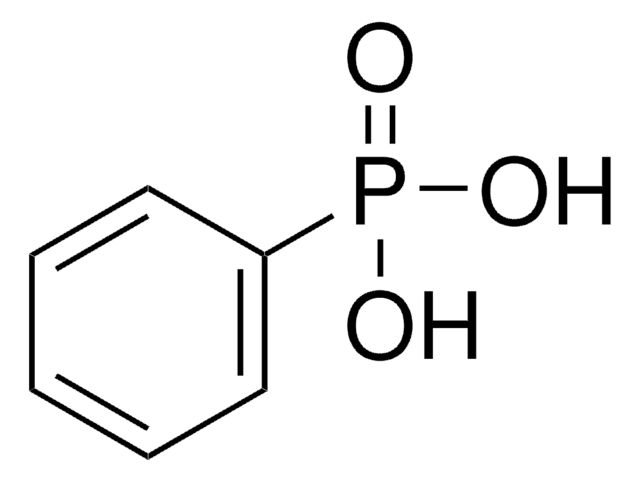
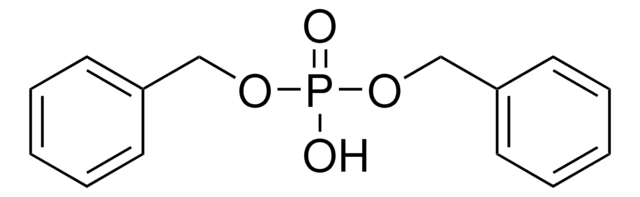
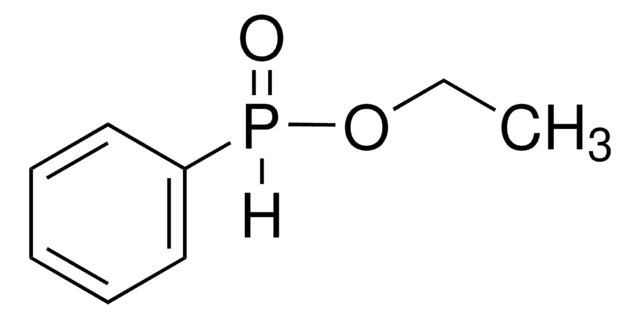
Diphenylphosphinic acid
≥98.0% (T)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(C6H5)2P(O)OH
Número de CAS:
Peso molecular:
218.19
Beilstein/REAXYS Number:
2804567
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥98.0% (T)
mp
193-195 °C (lit.)
193-196 °C
solubility
0.1 M NaOH: soluble 0.5 g/10 mL, clear, colorless
SMILES string
OP(=O)(c1ccccc1)c2ccccc2
InChI
1S/C12H11O2P/c13-15(14,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H,13,14)
InChI key
BEQVQKJCLJBTKZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Diphenylphosphinic acid (DPPA, hdpp) is an organophosphinic acid compound. It finds application as a reactive flame-retardant and promoter for palladium catalytic systems. Thermal degradation of DPPA has been studied by thermogravimetric analysis (TGA) method. DPPA reacts with cadmium nitrate in dimethylformamide solvent to afford the one-dimensional coordination polymer catena-poly[[bis(dimethylformamide-κO)cadmium(II)]-bis(μ-diphenylphosphinato-κ(2)O:O′)]. It has been repoted to promote the carbonylation of nitrobenzene and aniline to diphenylurea. Ortho-lithiated DPPA can form mono ortho-functionalized derivatives by electrophilic trapping and biphenyl-2,2′-diylbis(phenylphosphinic acid) by copper catalyzed coupling.
Application
Diphenylphosphinic acid is an important starting material for the synthesis of organophosphinic compounds. Diphenylphosphinic acid may be used for the preparation of coordination polymers, via reaction with alkaline earth metal salts in dimethylformamide (DMF) solvent:
- [Mg3(O2PPh2)6(DMF)2]·2DMF
- [Ca(O2PPh2)2(DMF)2]
- [Sr(O2PPh2)2(DMF)2]
- [Ba(O2PPh2)2(DMF)2]
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Jeffrey A Rood et al.
Acta crystallographica. Section C, Structural chemistry, 70(Pt 11), 1069-1074 (2014-11-06)
Reaction of cadmium nitrate with diphenylphosphinic acid in dimethylformamide solvent yielded the one-dimensional coordination polymer catena-poly[[bis(dimethylformamide-κO)cadmium(II)]-bis(μ-diphenylphosphinato-κ(2)O:O')], [Cd(C12H10O2P)2(C3H7NO)2]n, (I). Addition of 4,4'-bipyridine to the synthesis afforded a two-dimensional extended structure, poly[[(μ-4,4'-bipyridine-κ(2)N:N')bis(μ-diphenylphosphinato-κ(2)O:O')cadmium(II)] dimethylformamide monosolvate], {[Cd(C12H10O2P)2(C10H8N2)]·C3H7NO}n, (II). In (II), the 4,4'-bipyridine molecules link
A novel diphenylphosphinic acid coadsorbent for dye-sensitized solar cell.
Shen H, et al.
Electrochimica Acta, 56(5), 2092-2097 (2011)
Jeffrey A Rood et al.
Acta crystallographica Section B, Structural science, crystal engineering and materials, 70(Pt 3), 602-607 (2014-06-04)
Reaction of alkaline earth metal salts with diphenylphosphinic acid in dimethylformamide solvent afforded four coordination polymers: [Mg3(O2PPh2)6(DMF)2]·2DMF (I), [Ca(O2PPh2)2(DMF)2] (II), [Sr(O2PPh2)2(DMF)2] (III) and [Ba(O2PPh2)2(DMF)2] (IV) (where DMF is N,N-dimethylformamide). Single-crystal X-ray diffraction revealed that all four compounds produce linear chain
Thermal kinetics and decomposition mechanism of methylphenylphosphinic acid and diphenylphosphinic acid.
Shao X, et al.
Chemical Research in Chinese Universities, 30(6), 1028-1031 (2014)
Pengfei Hao et al.
Dalton transactions (Cambridge, England : 2003), 41(43), 13520-13524 (2012-09-29)
The reactions of LAlH2 (L = HC(CMeNAr)2, Ar = 2,6-iPr2C6H3) (1) with diphenylsilanediol, phenylphosphonic acid, diphenylphosphinic acid, and pyrocatechol afford compounds with the Al–O–X (X = Si, P, C) motif of composition [LAl(μ-O)]2Si(Ph)2 (2), [LAl(μ-O)]2PO(Ph) (3), LAl[OPO(Ph)2]2 (4), and LAl(μ-O)2(o-C6H4)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico