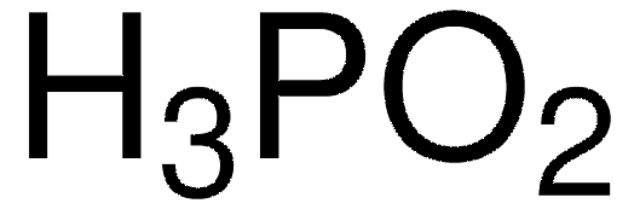P28808
Phenylphosphinic acid
99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5P(O)(OH)H
Número de CAS:
Peso molecular:
142.09
Beilstein/REAXYS Number:
2802244
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
form
powder
mp
83-85 °C (lit.)
SMILES string
O[PH](=O)c1ccccc1
InChI
1S/C6H7O2P/c7-9(8)6-4-2-1-3-5-6/h1-5,9H,(H,7,8)
InChI key
MLCHBQKMVKNBOV-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Phenylphosphinic acid is also known as phenylphosphonous acid. Kinetics of oxidation of phenylphosphinic acid by metal and non metal oxidants was investigated in a study.2
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
C Gervais et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 187(1), 131-140 (2007-05-08)
The complete set of NMR parameters for (17)O enriched phenylphosphinic acid C(6)H(5)HP( *)O(*OH) is calculated from first principles by using the Gauge Including Projected Augmented Wave (GIPAW) approach [C.J. Pickard, F. Mauri, All-electron magnetic response with pseudopotentials: NMR chemical shifts
Kunio Ikemura et al.
Dental materials journal, 28(3), 267-276 (2009-08-11)
The behavior of water-soluble photoinitiators with crown ethers in dental adhesives is unknown. This study investigated the effect of sodium acylphosphine oxide (APO-Na) with crown ether in a hydrophobic adhesive on adhesion to teeth. Sodium 2,4,6-trimethylbenzoyl-phenylphosphine oxide (TMPO-Na = APO-Na)
Li-Biao Han et al.
The Journal of organic chemistry, 70(24), 10121-10123 (2005-11-19)
[reaction: see text] A variety of functionalized optically pure (R(P))-alkylphenylphosphinates are readily prepared by stereospecific radical or base-catalyzed additions of the easily available (R(P))-menthyl phenylphosphinate to alkenes.
Loïc J Charbonnière et al.
Inorganic chemistry, 44(20), 7151-7160 (2005-09-27)
A new ligand, LC, bis-[(6'-carboxy-2,2'-bipyridine-6-yl)]phenylphosphine oxide, in which the tridentate 6-carboxy-2,2'-bipyridyl arms are directly linked to a phenylphosphine oxide fragment, has been synthesized. The corresponding [Ln.LC]Cl.xH2O complexes (Ln = Eu, x = 4, and Tb, x = 3) were isolated
E Maria Donner et al.
Drug and chemical toxicology, 26(2), 125-133 (2003-06-21)
Phenylphosphinic acid was fed in graded concentrations of either 0 (control), 100, 1,000, or 10,000 ppm to rats for 28 days. These concentrations provided average daily doses of 8, 76, and 779 mg/kg (males) and 9, 83, and 859 mg/kg
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico