08714
Methyl Red solution
suitable for microbiology
Synonym(s):
Methyl red indicator solution
About This Item
Recommended Products
Agency
according to GB 4789.30-2016
according to ISO 22964:2017
Quality Level
product line
BioChemika
shelf life
limited shelf life, expiry date on the label
composition
dist. water, 200 mL
ethanol 95%, 300 mL
methyl red, 0.1 g
technique(s)
microbe id | metabolite detection: suitable
application(s)
clinical testing
environmental
food and beverages
microbiology
suitability
Enterococcus spp.
Escherichia coli
Klebsiella spp.
Proteus spp.
Pseudomonas spp.
Staphylococcus spp.
bacteria
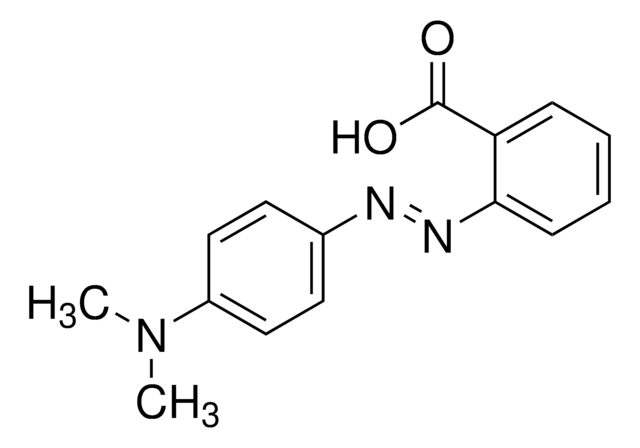
SMILES string
CN(C)c1ccc(cc1)\N=N\c2ccccc2C(O)=O
InChI
1S/C15H15N3O2/c1-18(2)12-9-7-11(8-10-12)16-17-14-6-4-3-5-13(14)15(19)20/h3-10H,1-2H3,(H,19,20)/b17-16+
InChI key
CEQFOVLGLXCDCX-WUKNDPDISA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
69.8 °F
Flash Point(C)
21 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
For microbiologists the most fundamental stain was developed in 1884 by the Danish bacteriologist Hans Christian Gram.
Sigma-Aldrich.com presents an article concerning Differentiation of Escherichia coli from coliforms.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service