06676
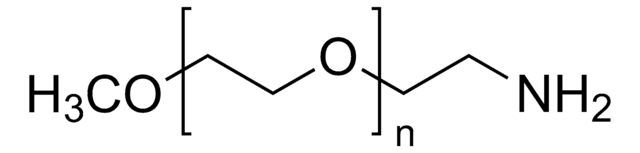
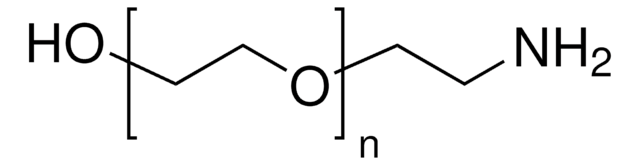
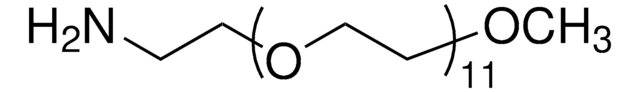
Methoxypolyethylene glycol amine
2,000, extent of labeling: ≥0.4 mmol/g NH2 loading
Synonym(s):
O-(2-Aminoethyl)-O′-methylpolyethylene glycol, Aminopolyethylene glycol monomethyl ether, Methoxypolyoxyethylene amine
About This Item
Recommended Products
Quality Level
form
powder
extent of labeling
≥0.4 mmol/g NH2 loading
InChI
1S/C3H9NO/c1-5-3-2-4/h2-4H2,1H3
InChI key
ASUDFOJKTJLAIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- As a supporting material in the synthesis of polymer-lipid conjugates.
- As an initiator for ring-opening polymerization (ROP) of N-substituted N-carboxy anhydrides (NNCAs) to prepare polypeptoids.
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Accumulation of biological matter at surfaces is an inevitable event in virtually any environment in which natural and man-made materials are used. Although sometimes fouling of surfaces with biomolecules and bioorganisms has little consequence, biofouling must be minimized or controlled in order to maintain performance and safety of devices and structures.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service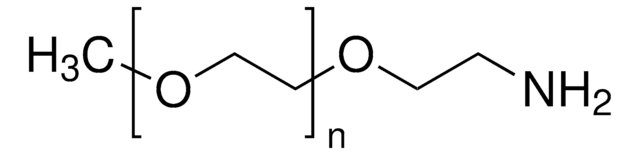
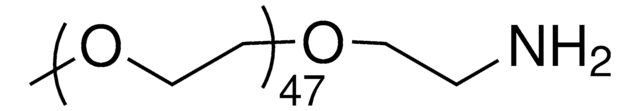

![O-[(N-Succinimidyl)succinyl-aminoethyl]-O′-methylpolyethylene glycol 2′000](/deepweb/assets/sigmaaldrich/product/structures/426/156/518cf3f3-8b50-4c91-8501-929224a040d2/640/518cf3f3-8b50-4c91-8501-929224a040d2.png)