QBD10776
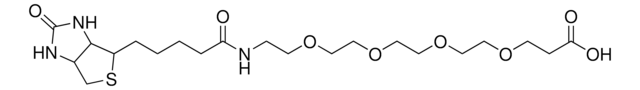
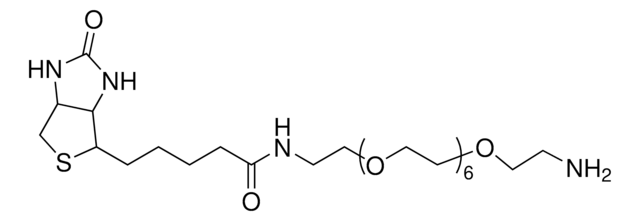
dPEG®48-biotin acid
>90% (HPLC)
Synonym(s):
Biotin-PEG-acid, Biotin-PEG48-COOH, PEG2000 biotin acid, PEG48-biotin acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C112H218N4O53S
Molecular Weight:
2500.99
UNSPSC Code:
12352106
NACRES:
NA.22
Recommended Products
Assay
>90% (HPLC)
form
solid or viscous liquid
reaction suitability
reaction type: Biotinylations
reaction type: Pegylations
polymer architecture
shape: linear
functionality: monofunctional
shipped in
ambient
storage temp.
−20°C
Features and Benefits
dPEG48 biotin acid is a 2,500 Dalton biotin acid product that contains a 157 atom (187.8 Å) dPEG spacer. Amphiphilic dPEG48 biotin acid dissolves in water or aqueous buffer and organic solvents. It does not cause aggregation or precipitation of biomolecules. Using 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) chemistry, dPEG48 biotin acid couples to free primary amines in aqueous media. These free amines can be on a protein, peptide, or the treated surface of a nanoparticle. Moreover, the terminal propionic acid moiety can be functionalized with other reactive groups for coupling directly to surfaces such as glass, gold, or magnetic nanoparticles. dPEG48 biotin acid is a monodisperse equivalent of the polymeric PEG2000.
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Legal Information
Products Protected under U.S. Patent #s 7,888,536 & 8,637,711 and European Patent #s 1,594,440 & 2,750,681
dPEG is a registered trademark of Quanta BioDesign
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thurid Boetel et al.
Biochemical and biophysical research communications, 349(1), 296-302 (2006-08-29)
The biological function of prion protein (PrP) and the physiological relevance of its truncated subtypes and glycoforms is still enigmatic. In this paper, we adduce evidence that recombinant murine PrP fragment 90-231 (mPrP90-231) contains a biotin-mimicking sequence motif that causes
Alexander Kuzmin et al.
Bioconjugate chemistry, 21(11), 2076-2085 (2010-10-23)
The utility of catalyst-free azide-alkyne [3 + 2] cycloaddition for the immobilization of a variety of molecules onto a solid surface and microbeads was demonstrated. In this process, the surfaces are derivatized with aza-dibenzocyclooctyne (ADIBO) for the immobilization of azide-tagged
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


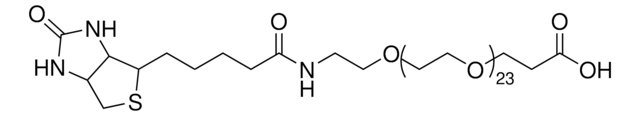

![O-[2-(Biotinylamino)ethyl]-O′-(2-carboxyethyl)undecaethylene glycol ≥95% (oligomer purity)](/deepweb/assets/sigmaaldrich/product/structures/445/096/aadb4590-ea90-4e6c-835d-a9aef2828e9a/640/aadb4590-ea90-4e6c-835d-a9aef2828e9a.png)