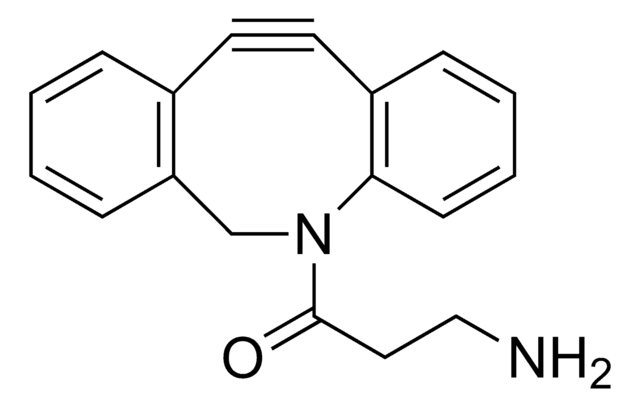
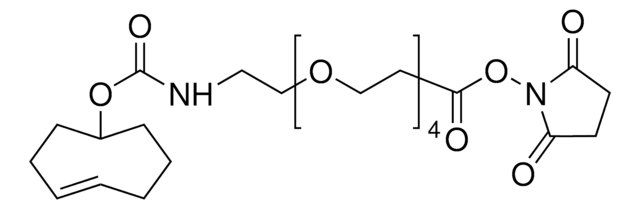
900913
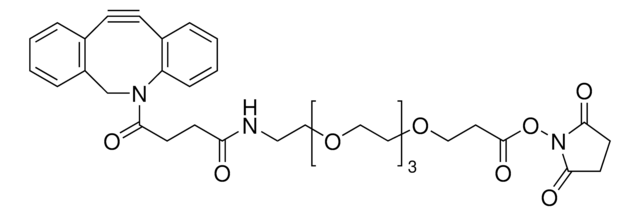
Tetrazine-PEG5-NHS ester
≥95%
Synonym(s):
Tz-PEG5-NHS
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C27H36N6O10
CAS Number:
Molecular Weight:
604.61
MDL number:
UNSPSC Code:
12352108
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
powder or crystals
reaction suitability
reaction type: click chemistry
reagent type: linker
functional group
NHS ester
storage temp.
−20°C
SMILES string
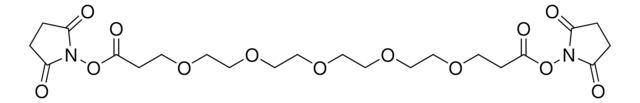
O=C(NCC1=CC=C(C2=NN=CN=N2)C=C1)CCOCCOCCOCCOCCOCCC(ON3C(CCC3=O)=O)=O
General description
Tetrazine-PEG5-NHS ester has an N-hydroxysuccinimide (NHS) ester that can be reacted with primary amines and tetrazine, which is reactive to trans-cyclooctenes. Hydrogen substituted tetrazines demonstrate exceptionally fast kinetics, generally at least 10-fold faster compared to methyl substituted tetrazines. The aqueous solubility of this reagent is substantially enhanced by a hydrophilic polyethylene glycol (PEG) spacer arm.
Application
Tetrazine-PEG5-NHS ester (Tz-PEG5-NHS) can be used in click chemistry applications such as the preparation of chitosan-polyacrylamide microspheres utilizing selective tetrazine-trans-cyclooctene (Tz-TCO) ligation and the construction of copper-64 radiolabeled antibody by the click reaction.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shape-Encoded Chitosan?Polyacrylamide Hybrid Hydrogel Microparticles with Controlled Macroporous Structures via Replica Molding for Programmable Biomacromolecular Conjugation.
Kang E, et al.
Langmuir, 32(21), 5394-5402 (2016)
Porosity-Tuned Chitosan?Polyacrylamide Hydrogel Microspheres for Improved Protein Conjugation.
Jung S, et al.
Biomacromolecules, 17(7), 2427-2436 (2016)
Amit Kumar et al.
Bioconjugate chemistry, 26(4), 782-789 (2015-03-12)
We report a click-chemistry based modular strategy for antibody labeling with (64)Cu (t1/2 = 12.7 h; β(+) 0.656 MeV, 17.4%; β(-) 0.573 MeV, 39%; EC 43%) under ambient condition utilizing a cross-bridged tetraazamacrocyclic (CB-TE2A) analogue, which otherwise requires harsh conditions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service