856061
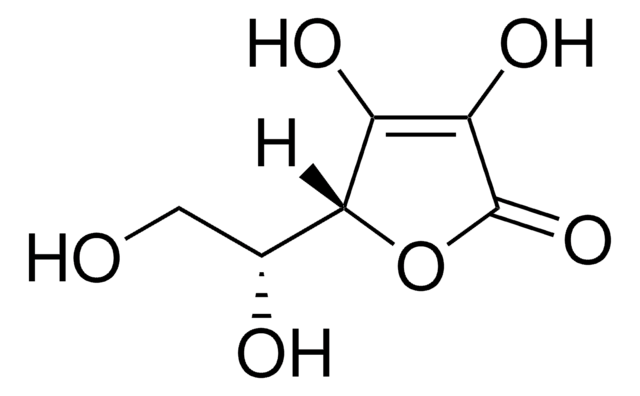
D-(−)-Isoascorbic acid
98%
Synonym(s):
D-erythro-Hex-2-enoic acid γ-lactone, D-Araboascorbic acid, Erythorbic acid, Glucosaccharonic acid, NSC 8117
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H8O6
CAS Number:
Molecular Weight:
176.12
Beilstein:
84271
EC Number:
MDL number:
UNSPSC Code:
12352205
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
optical activity
[α]25/D −16.8°, c = 2 in H2O
mp
169-172 °C (dec.) (lit.)
SMILES string
[H][C@@]1(OC(=O)C(O)=C1O)[C@H](O)CO
InChI
1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-10H,1H2/t2-,5-/m1/s1
InChI key
CIWBSHSKHKDKBQ-DUZGATOHSA-N
Looking for similar products? Visit Product Comparison Guide
General description
D-(−)-Isoascorbic acid, also known as erythorbic acid, is widely utilized as a chiral building block in organic synthesis for the preparation of various chiral compounds. It is also used as a reducing agent in various organic reactions.
Application
D-(−)-Isoascorbic acid can be used as a reactant in the synthesis of various chiral compounds such as:
- enantiopure aminotriol
- (3R, 4S)-4-hydroxylasiodiplodin and D-mycinose
- enantiomerically pure stereoisomers of α,β-dihydroxy-aldehydes or acids
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andrew C Clark et al.
Journal of agricultural and food chemistry, 58(2), 1004-1011 (2009-12-31)
The stereochemical influence of antioxidant and flavanol compounds on oxidation processes in a model wine system was studied. The diastereoisomers, ascorbic acid and erythorbic acid, were used as antioxidants in a model wine system containing either (+)-catechin or (-)-epicatechin as
Manuel Bueno et al.
Carbohydrate research, 344(15), 2100-2104 (2009-07-17)
l-Ascorbic and d-isoascorbic acids have been used as the starting materials for the preparation of (3R,4'S)-3-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPTA), (3R and S, 4'S,6R)-3-methyl-6-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPTP) and (3R,4'R)-3-(2',2'-dimethyl-1',3'-dioxolan-4'-yl)-1,4-dioxane-2,5-dione (IPEA), three novel 1,4-dioxane-2,5-dione-type monomers. Ring-opening homopolymerisation and copolymerisation of the IPTA monomer, derived from l-ascorbic
Spyros Drivelos et al.
Analytical and bioanalytical chemistry, 397(6), 2199-2210 (2010-04-16)
A new hydrophilic interaction liquid chromatographic (HILIC) method for the simultaneous determination of isoascorbic (IAA) and ascorbic acid (AA) was developed. The separation of IAA and AA was studied in various HILIC stationary phases and the influence of the composition
Daiki Kyotani et al.
Bioscience, biotechnology, and biochemistry, 73(4), 954-956 (2009-04-09)
We found that a strain of Penicillium sp. effectively converted L-ascorbic acid to a five-carbon analog, which was identified as L-erythroascorbic acid based on spectroscopic analysis. The conversion was achieved by growing culture or washed mycelia, with a yield of
Alberto Baroja-Mazo et al.
Fungal genetics and biology : FG & B, 42(5), 390-402 (2005-04-06)
D-Erythroascorbate and D-erythroascorbate glucoside have been identified in the Zygomycete fungus Phycomyces blakesleeanus. Ascomycete and Basidiomycete fungi also synthesise D-erythroascorbate instead of l-ascorbate, suggesting that D-erythroascorbate synthesis evolved in the common ancestor of the fungi. Both compounds accumulate in P.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service