729078
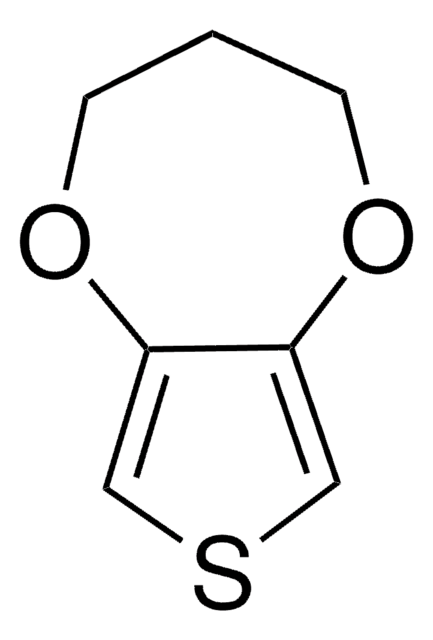
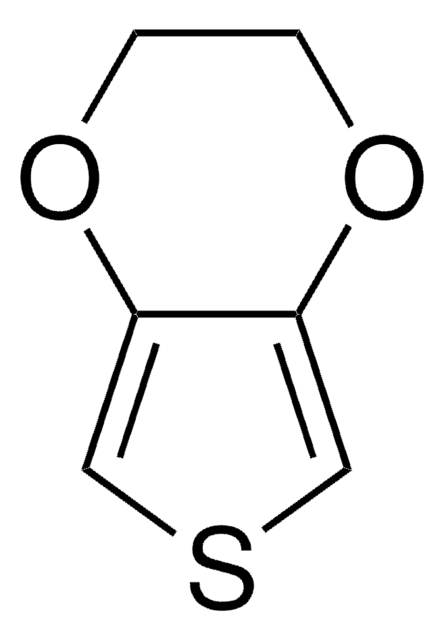
3,4-Ethylenedithiothiophene
Synonym(s):
3,4-Ethylenedithiothiophene, 2,3-Dihydrothieno[3,4-b][1,4]dithiine, 3,4-Ethylenedisulfanylthiophene, EDTT
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H6S3
CAS Number:
Molecular Weight:
174.31
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
liquid
refractive index
n20/D 1.685
density
1.374 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
C1CSc2cscc2S1
InChI
1S/C6H6S3/c1-2-9-6-4-7-3-5(6)8-1/h3-4H,1-2H2
InChI key
HPGNGICCHXRMIP-UHFFFAOYSA-N
General description
3,4-Ethylenedithiothiophene (EDTT) is a dithiin based conducting polymer that is a sulfur analog of 3,4-ethylendioxythiophene (EDOT) that is majorly used in optoelectronic and electrochemical devices.
Application
EDTT can be electrochemically oxidized in acetonitrile to form poly(EDTT) films on the surface of the electrodes, which can be used in the fabrication of organic light emitting diodes (OLEDs) and organic photovoltaics (OPVs).
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mathieu, T.; et al.
Macromolecules, 38, 6806-6812 (2005)
Functionalized 3, 4-ethylenedithiathiophenes (EDTTs) as building blocks for poly (3, 4-ethylenedithiathiophene)(PEDTT) derivatives.
Blanco R, et al.
Tetrahedron Letters, 49(13), 2056-2059 (2008)
Randriamahazaka, H.; Sini, G.; Tran Van, F.
The Journal of Physical Chemistry C, 111, 4553-4560 (2007)
Redox doping behaviour of poly (3, 4-ethylenedithiothiophene)-The counterion effect.
Domagala W, et al.
Optical Materials, 33(9), 1405-1409 (2011)
Thierry Darmanin et al.
Journal of colloid and interface science, 466, 413-424 (2016-01-16)
Controlling the formation of surface nanostructures and nanotubes in particular is extremely important for various applications in electronic devices for energy systems, biosensing but also for the control of water adhesion. Here, we use a direct (without template) electropolymerization process
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service