All Photos(2)
About This Item
Empirical Formula (Hill Notation):
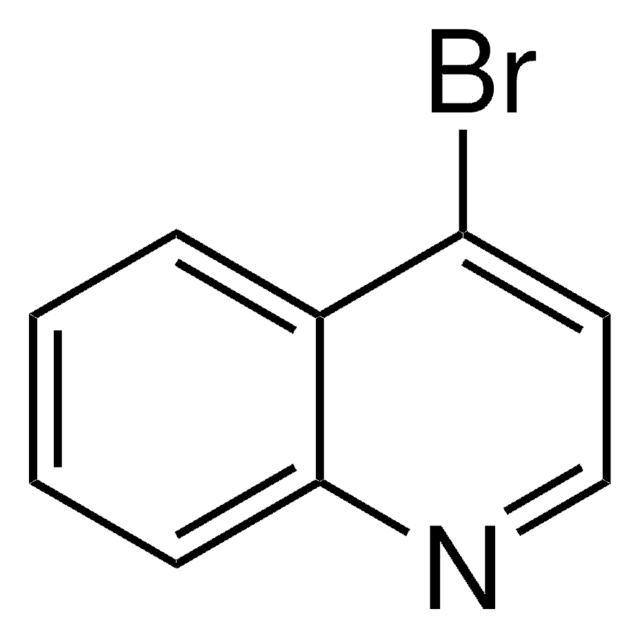
C9H6BrN
CAS Number:
Molecular Weight:
208.05
Beilstein:
112939
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.664 (lit.)
bp
274-276 °C (lit.)
mp
13-15 °C (lit.)
density
1.533 g/mL at 25 °C (lit.)
SMILES string
Brc1cnc2ccccc2c1
InChI
1S/C9H6BrN/c10-8-5-7-3-1-2-4-9(7)11-6-8/h1-6H
InChI key
ZGIKWINFUGEQEO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Bromoquinoline undergoes bromine-magnesium exchange reaction with lithium tributylmagnesate in toluene at -10°C, which is quenched by various electrophiles to yield functionalized quinolines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tributylmagnesium ate complex-mediated bromine-magnesium exchange of bromoquinolines: a convenient access to functionalized quinolines.
Dumouchel S, et al.
Tetrahedron Letters, 44(10), 2033-2035 (2003)
Patrick W Fedick et al.
Journal of the American Society for Mass Spectrometry, 30(10), 2144-2151 (2019-08-09)
Suzuki cross-coupling is a widely performed reaction, typically using metal catalysts under heated conditions. Acceleration of the Suzuki cross-coupling reaction has been previously explored in microdroplets using desorption electrospray ionization mass spectrometry (DESI-MS). Building upon previous work, presented here is
Bin Yang et al.
Environmental science and pollution research international, 23(4), 3399-3405 (2015-10-23)
The solubilities of 19 different kinds of N-heteroaromatic compounds in aqueous solutions with different concentrations of NaCl were determined at 298.15 K with a UV-vis spectrophotometry and titration method, respectively. Setschenow constants, Ks, were employed to describe the solubility behavior
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service