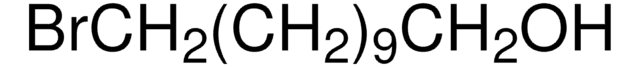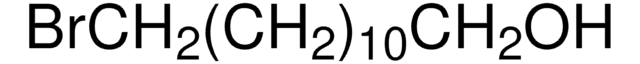All Photos(1)
About This Item
Linear Formula:
Br(CH2)10CH2SH
CAS Number:
Molecular Weight:
267.27
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.501
density
1.128 g/mL at 25 °C
storage temp.
−20°C
SMILES string
SCCCCCCCCCCCBr
InChI
1S/C11H23BrS/c12-10-8-6-4-2-1-3-5-7-9-11-13/h13H,1-11H2
InChI key
IKVISLWYYHGTTN-UHFFFAOYSA-N
General description
11-Bromo-1-undecanethiol (BrUDT) is a bromo-terminated alkanethiol that forms a self-assembled monolayer(SAM) on a variety of surfaces. It can modify the surface characteristics by forming a bond between the sulfur groups and surface atoms.
Application
BrUDT forms a protective SAM on gold nanoparticles, which can be potentially used in biomedical applications for the fabrication of in vivo sensors. It can also be mixed with dodecanethiol, which can be coated on gold nanoparticles for the synthesis of gasotransmitters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Delivering nitric oxide with nanoparticles.
Quinn JF, et al.
Journal of Controlled Release : Official Journal of the Controlled Release Society, 205(26), 190-205 (2015)
Remarkable high-yielding chemical modification of gold nanoparticles using uncatalyzed click-type 1, 3-dipolar cycloaddition chemistry and hyperbaric conditions.
Ismaili H, et al.
Canadian Journal of Chemistry, 87(12), 1708-1715 (2009)
Georgios Stratis et al.
The Journal of chemical physics, 154(3), 034704-034704 (2021-01-28)
The breaking of molecular bonds during exposure to ionizing radiation and electron beams creates irreversible damage in the molecular structure. In some cases, such as lithography, controlled damage of a molecular resist is a desirable process and is the basis
Direct and label-free influenza virus detection based on multisite binding to sialic acid receptors.
Yukichi Horiguchi et al.
Biosensors & bioelectronics, 92, 234-240 (2017-02-22)
A system to discriminate human or avian influenza A remains a highly sought-after tool for prevention of influenza pandemics in humans. Selective binding of the influenza A viral hemagglutinin (HA) to specific sialic acid (SA) receptors (Neu5Acα(2-6)Gal in humans, Neu5Acα(2-3)Gal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service