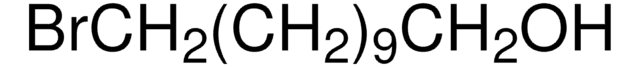467642
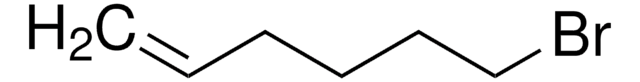
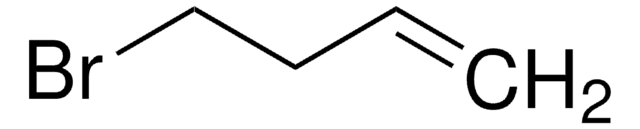
11-Bromo-1-undecene
95%
Synonym(s):
1-Bromo-10-undecene, 10-Undecenyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)9CHCH2
CAS Number:
Molecular Weight:
233.19
Beilstein:
1753231
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.468 (lit.)
bp
149-150 °C/35 mmHg (lit.)
density
1.063 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCCCCC=C
InChI
1S/C11H21Br/c1-2-3-4-5-6-7-8-9-10-11-12/h2H,1,3-11H2
InChI key
YPLVPFUSXYSHJD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
11-Bromo-1-undecene is a halogenated hydrocarbon. It can be synthesized by employing alkenyl esters or dibromides as starting materials.
Application
11-Bromo-1-undecene to synthesize brominated polyethylene (PE) via metallocene-catalyzed copolymerization with ethylene. It is also used to prepare poly(olefin)-based anion exchange membranes (AEMs) for practical alkaline fuel cell applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The facile preparation of alkenyl metathesis synthons.
Baughman TW, et al.
Tetrahedron, 60(48), 10943-10948 (2004)
Polymer Preprints (American Chemical Society, Division of Polymer Chemistry), 45, 931-932 (2004)
Marrani AG, et al.
Electrochimica Acta, 53(11), 3903-3909 (2008)
Synthesis of epoxy-terminated dialkyl disulfides for polymerizable self-assembled monolayers.
Yeager LJ, et al.
Tetrahedron Letters, 39(46), 8409-8412 (1998)
Covalent and Non-covalent Attachment and Patterning of Polypyrrole at Silicon Surfaces.
Pike AR, et al.
Advanced Materials, 15(3), 254-257 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service