56210
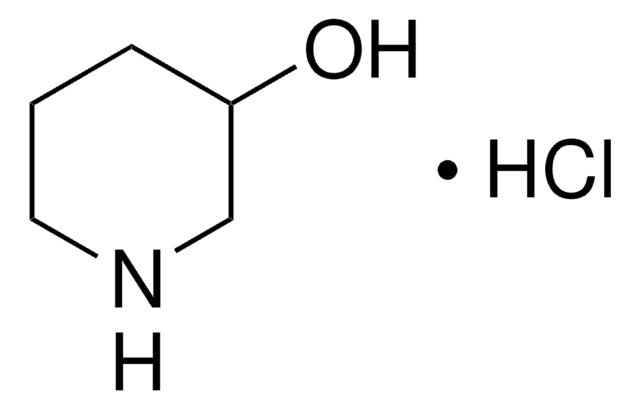
3-Hydroxypiperidine
≥98.0% (NT)
Synonym(s):
3-Piperidinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H11NO
CAS Number:
Molecular Weight:
101.15
Beilstein:
102696
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (NT)
form
solid
mp
57-61 °C
solubility
H2O: 1 g/mL, clear
storage temp.
2-8°C
SMILES string
OC1CCCNC1
InChI
1S/C5H11NO/c7-5-2-1-3-6-4-5/h5-7H,1-4H2
InChI key
BIWOSRSKDCZIFM-UHFFFAOYSA-N
General description
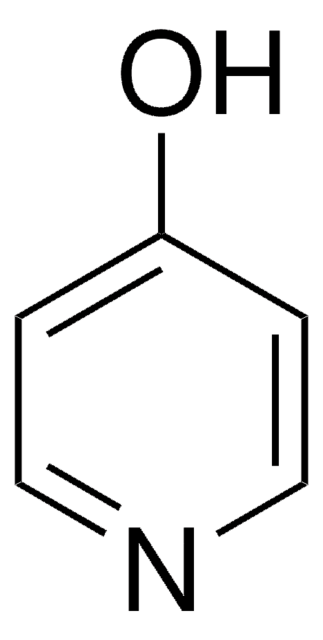
3-Hydroxypiperidine can be obtained from the reduction of 3-hydroxypyridine.
Application
3-Hydroxypiperidine may be used to synthesize the following:
- 2-pyrrolidinone via oxidation with iodosylbenzene
- 1-[2,8-bis(trifluoromethyl)quinolin-4-yl]piperidin-3-ol via reaction with 4-bromoquinoline
Reactant for:
Synthesis of unsymmetrical ureas
Synthesis of piperidine nucleoside analogs
Fluorination reactions
Synthesis of substituted pyridines
Synthesis of unsymmetrical ureas
Synthesis of piperidine nucleoside analogs
Fluorination reactions
Synthesis of substituted pyridines
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Katritzky.RA, et al.
Comprehensive Organic Functional Group Transformations II, 1 (1995)
Synthesis of quinoline analogs: search for antimalarial agents
Babu R K, et al.
Monatshefte fur Chemie / Chemical Monthly, 139(2), 179-181 (2008)
Oxidation of 3-hydroxypiperidines with iodosylbenzene in water: Tandem oxidative Grob fragmentation-cyclization reaction
Tada N, et al.
Chemical & Pharmaceutical Bulletin, 52(9), 1143-1144 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service