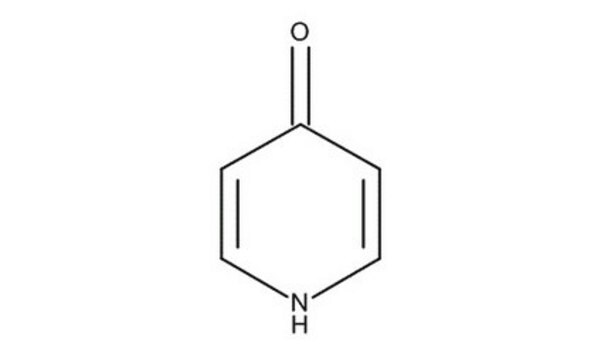
120618
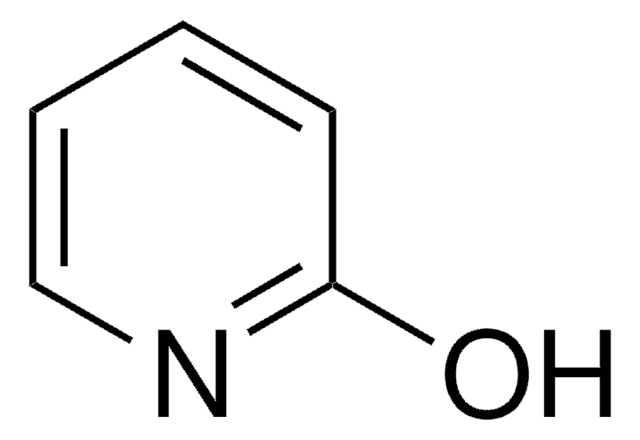
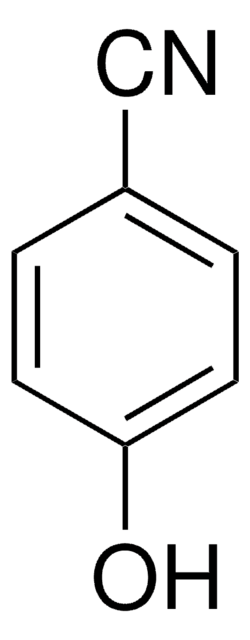
4-Hydroxypyridine
95%
Synonym(s):
4-Pyridinol, 4-Pyridone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105800
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
bp
230-235 °C/12 mmHg (lit.)
mp
150-151 °C (lit.)
SMILES string
O=C1C=CNC=C1
InChI
1S/C5H5NO/c7-5-1-3-6-4-2-5/h1-4H,(H,6,7)
InChI key
GCNTZFIIOFTKIY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Hydroxypyridine was used in the synthesis of (Ag3MoO3F3) (Ag3MoO4)Cl by hydro(solvato)thermal methods. It was used as model compound to study the natural photodegradation of representative aquatic environmental contaminants.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Natsuki K Kubota et al.
Bioorganic & medicinal chemistry, 11(21), 4569-4575 (2003-10-07)
Piericidins C5 (1) and C6 (2), two new members of the piericidin family, were isolated from a Streptomyces sp. and a Nocardioides sp., together with known piericidins C1 (3), C2 (4), C3 (5), C4 (6), D1 (7), and A3 (8).
Xiaoqing Cai et al.
Bioorganic & medicinal chemistry, 20(11), 3584-3595 (2012-05-09)
Bicyclic pyridinol antioxidants have been reported to suppress the autoxidation of methyl linoleate more effectively than α-tocopherol in benzene solution. A few novel lipophilic analogues have recently been synthesized by conjugating a pyridinol core with the phytyl side chain of
Alignment of acentric MoO3F33-anions in a polar material :(AgMoO3F3)(Ag3MoO4) Cl.
Maggard PA, et al.
Journal of Solid State Chemistry, 175(1), 27-33 (2003)
Heiko Zettl et al.
ACS chemical biology, 7(9), 1488-1495 (2012-06-26)
We present an integrated approach to identify and optimize a novel class of γ-secretase modulators (GSMs) with a unique pharmacological profile. Our strategy included (i) virtual screening through application of a recently developed protocol (PhAST), (ii) synthetic chemistry to discover
H Chen et al.
Biochemistry, 32(43), 11591-11599 (1993-11-02)
We have examined the interaction of Citrobacter freundii tyrosine phenol-lyase with both L- and D-alanine. This enzyme catalyzes the racemization of alanine as a side reaction, in addition to the physiological beta-elimination of L-tyrosine to give phenol and ammonium pyruvate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service