55452
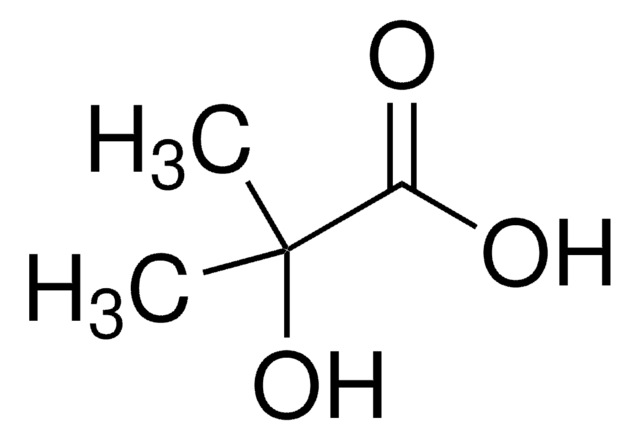
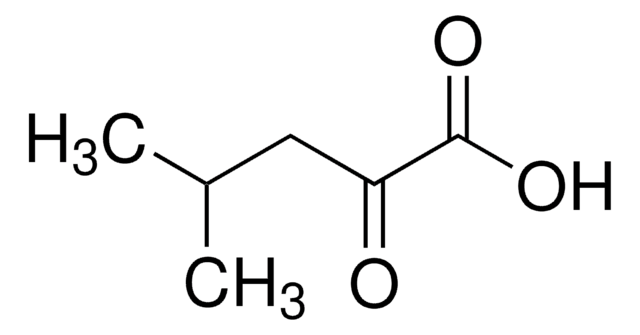
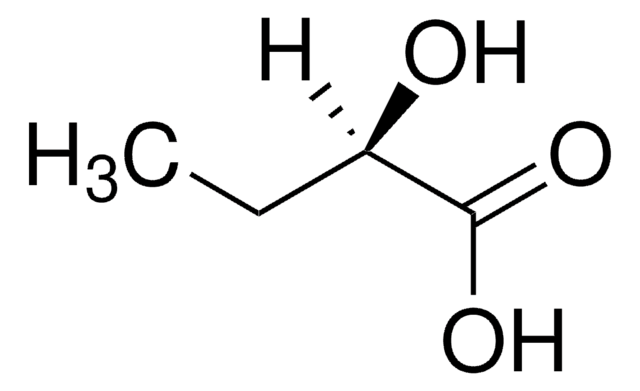
D-α-Hydroxyisovaleric acid
≥98.0% (T)
Synonym(s):
(R)-2-Hydroxy-3-methylbutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H10O3
CAS Number:
Molecular Weight:
118.13
Beilstein:
1721139
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (T)
form
solid
optical activity
[α]20/D −17±1°, c = 1% in chloroform
mp
64-67 °C
functional group
carboxylic acid
hydroxyl
SMILES string
CC(C)[C@@H](O)C(O)=O
InChI
1S/C5H10O3/c1-3(2)4(6)5(7)8/h3-4,6H,1-2H3,(H,7,8)/t4-/m1/s1
InChI key
NGEWQZIDQIYUNV-SCSAIBSYSA-N
Application
D-α-Hydroxyisovaleric acid may be used in the preparation of biodegradable, optically active and isotactic poly(D-2-hydroxy-3-methylbutanoic acid).
Other Notes
This chiral α-hydroxy acid is used for the synthesis of peptides and depsipeptides. It is further used as chiral building block. Synthesis of α-substituted α-hydroxy acids via dioxolanone intermediates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hetero-stereocomplex formation between substituted poly (lactic acid) s with linear and branched side chains, poly (l-2-hydroxybutanoic acid) and poly (d-2-hydroxy-3-methylbutanoic acid).
Tsuji H and Hayakawa T.
Polymer, 55(3), 721-726 (2014)
W. Hartwig et al.
Liebigs Ann. Chem., 1952-1952 (1982)
H.-O. Kim et al.
The Journal of Organic Chemistry, 52, 4531-4531 (1987)
R L Johnson
Journal of medicinal chemistry, 23(6), 666-669 (1980-06-01)
The following N-(alpha-hydroxylakanoyl) derivatives of Leu-Val-Phe-OCH3 were synthesized and tested for their ability to inhibit human amniotic renin: D- and L-alpha-hydroxyisocaproyl-Leu-Val-Phe-OCH3, D- and L-alpha-hydroxyisovaleryl-Leu-Val-Phe-OCH3, L-2-hydroxy-3-phenylpropanoyl-Leu-Val-Phe-OCH3, and D- and L-alpha-hydroxyphenylacetyl-Leu-Val-Phe-OCH3. Analysis of the compounds through the use of Dixion plots showed
Hye-Ran Yoon
Archives of pharmacal research, 30(3), 387-395 (2007-04-12)
A rapid dried-filter paper plasma-spot analytical method was developed to quantify organic acids, amino acids, and glycines simultaneously in a two-step derivatization procedure with good sensitivity and specificity. The new method involves a two-step trimethylsilyl (TMS) - trifluoroacyl (TFA) derivatization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service