68255
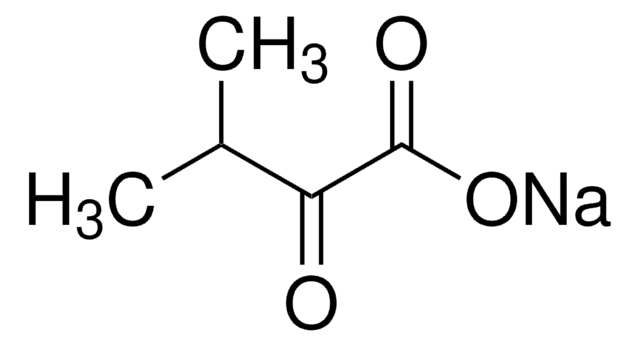
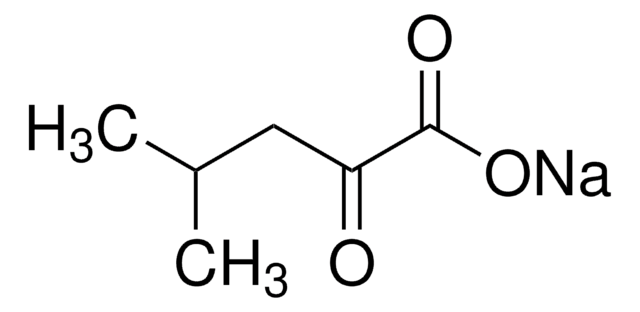
4-Methyl-2-oxovaleric acid
≥98.0% (T)
Synonym(s):
α-Ketoisocaproic acid, 2-Oxoisocaproic acid, 4-Methyl-2-oxopentanoic acid, Ketoleucine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2CHCH2COCOOH
CAS Number:
Molecular Weight:
130.14
Beilstein:
1701823
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (T)
refractive index
n20/D 1.431
bp
82-83 °C/11 mmHg (lit.)
mp
8-10 °C
density
1.055 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)CC(=O)C(O)=O
InChI
1S/C6H10O3/c1-4(2)3-5(7)6(8)9/h4H,3H2,1-2H3,(H,8,9)
InChI key
BKAJNAXTPSGJCU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Methyl-2-oxovaleric acid, also known as α-ketoisocaproic acid (KIC), is a metabolite of leucine. It is commonly used as a building block in the synthesis of leucine and its derivatives, and it plays a role in amination reactions and asymmetric synthesis. Additionally, KIC serves as an intermediate in the formation of Strecker aldehydes.
Application
- Role in Human Brain Ketometabolism: 4-Methyl-2-oxovaleric acid has been implicated in the temporal patterns of ketometabolism in cerebral microdialysis fluids of patients with traumatic brain injury, underscoring its significance in biochemical pathways within the brain (Eiden et al., 2019).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yulan Wang et al.
Proceedings of the National Academy of Sciences of the United States of America, 101(34), 12676-12681 (2004-08-18)
Schistosomiasis, a chronic and debilitating parasitic disease, affects approximately 200 million people in the developing world and imposes a substantial public health and economic impact. Accurately diagnosing at the individual level, monitoring disease progression, and assessing the impact of pharmacological
Lisa S Chow et al.
American journal of physiology. Endocrinology and metabolism, 291(4), E729-E736 (2006-05-18)
Despite being an anabolic hormone in skeletal muscle, insulin's anticatabolic mechanism in humans remains controversial, with contradictory reports showing either stimulation of protein synthesis (PS) or inhibition of protein breakdown (PB) by insulin. Earlier measurements of muscle PS and PB
D L Hachey et al.
Analytical chemistry, 63(9), 919-923 (1991-05-01)
A rapid, single-step procedure for the extraction and derivatization of organic alpha-keto acids from microliter quantities of human plasma has been developed. The keto acids were analyzed as the pentafluorobenzyl (PFB) ester by methane negative chemical ionization gas chromatography/mass spectrometry.
W E Mitch et al.
The Journal of clinical investigation, 67(2), 553-562 (1981-02-01)
We measured the effects of seven consecutive daily infusions of alpha-ketoisocaproate (the alpha-keto analogue of leucine) or leucine itself on urinary urea and total nitrogen excretion during fasting. Two study protocols were undertaken. In protocol I, subjects underwent three separate
Eiji Yoshihara et al.
Nature communications, 1, 127-127 (2010-12-02)
Type 2 diabetes mellitus (T2DM) is characterized by defects in both insulin sensitivity and glucose-stimulated insulin secretion (GSIS) and is often accompanied by obesity. In this study, we show that disruption of thioredoxin binding protein-2 (TBP-2, also called Txnip) in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service