All Photos(2)
About This Item
Linear Formula:
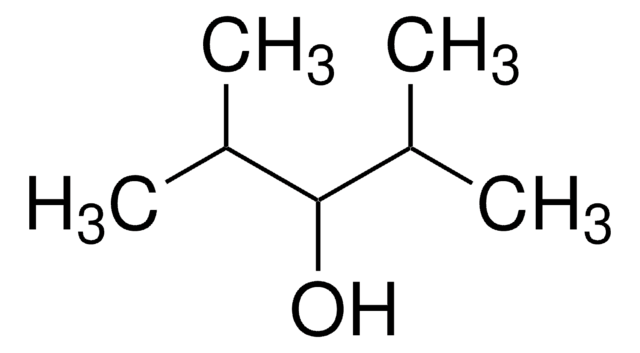
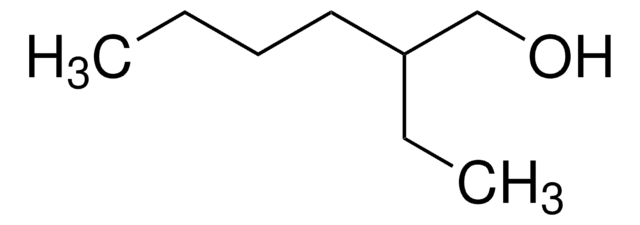
CH3CH(CH3)CH2CH(OH)CH2CH(CH3)CH3
CAS Number:
Molecular Weight:
144.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
80%
refractive index
n20/D 1.423 (lit.)
bp
178 °C (lit.)
density
0.809 g/mL at 25 °C (lit.)
SMILES string
CC(C)CC(O)CC(C)C
InChI
1S/C9H20O/c1-7(2)5-9(10)6-8(3)4/h7-10H,5-6H2,1-4H3
InChI key
HXQPUEQDBSPXTE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,6-Dimethyl-4-heptanol may be used in the preparation of the protected β-hydroxybutyrates. It may also be used as a hydrogen donor during the dynamic kinetic resolution (DKR) of various diols, monoprotected diols and the protected hydroxy aldehydes.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dynamic kinetic resolution of secondary alcohols by enzyme?metal combinations in ionic liquid.
Kim MJ, et al.
Green Chemistry, 6(9), 471-474 (2004)
Lipase/ruthenium-catalyzed dynamic kinetic resolution of hydroxy acids, diols, and hydroxy aldehydes protected with a bulky group.
M J Kim et al.
The Journal of organic chemistry, 66(13), 4736-4738 (2001-06-26)
Mahn Joo Kim et al.
Current opinion in biotechnology, 13(6), 578-587 (2002-12-17)
The combination of enzyme and metal catalysis is described as a useful method for the synthesis of optically active compounds. A key feature of this new methodology is the use of metal catalysts for the in situ racemization of enzymatically
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service