150045
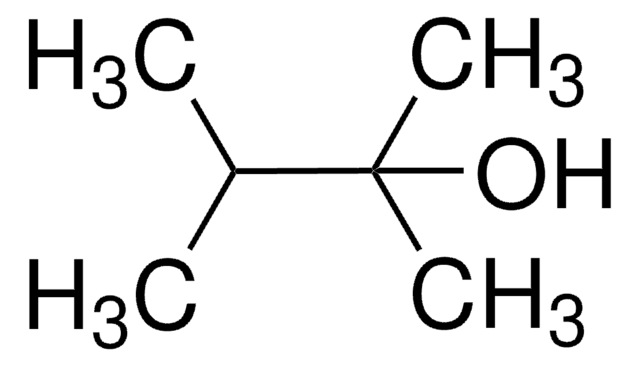
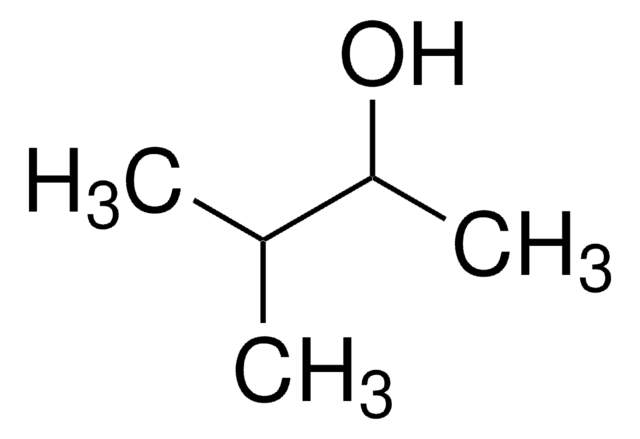
2,4-Dimethyl-3-pentanol
99%
Synonym(s):
Diisopropylcarbinol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2CHCH(OH)CH(CH3)2
CAS Number:
Molecular Weight:
116.20
Beilstein:
1731593
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
refractive index
n20/D 1.425 (lit.)
bp
139-140 °C (lit.)
density
0.829 g/mL at 25 °C (lit.)
SMILES string
CC(C)C(O)C(C)C
InChI
1S/C7H16O/c1-5(2)7(8)6(3)4/h5-8H,1-4H3
InChI key
BAYAKMPRFGNNFW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
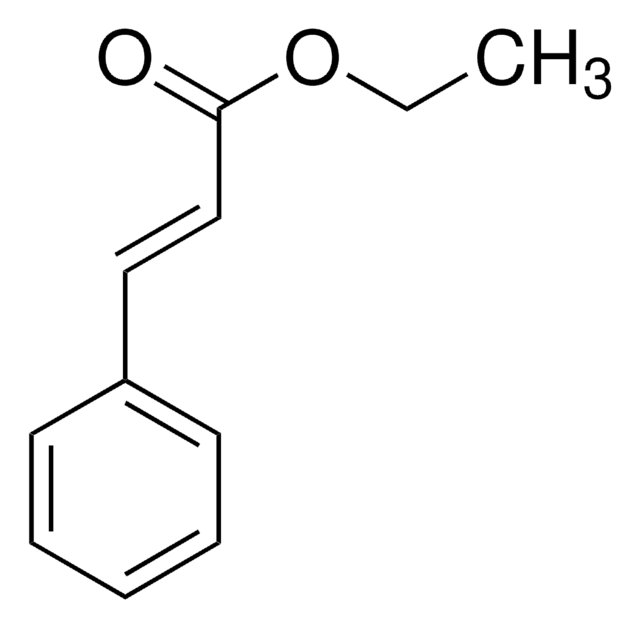
2,4-Dimethyl-3-pentanol was used as a proton source in the diastereoselective coupling with 2-substituted acrylate derivatives. It was used in the polymerization of 1,1′-(1,3-phenylene) diethanol (1,3-diol) and diisopropyl adipate.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
98.6 °F - closed cup
Flash Point(C)
37 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hegui Gong et al.
Organic letters, 11(4), 879-882 (2009-01-28)
A mild, stereoselective method for the Ni-catalyzed synthesis of alpha-C-alkylglycosides is reported. This approach entails the reductive coupling of glycosyl bromides with activated alkenes at room temperature, with low alkene loading as an important feature. Diastereoselective coupling with 2-substituted acrylate
Bart A C van As et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(29), 8325-8332 (2007-07-31)
The well-known dynamic kinetic resolution of secondary alcohols and esters was extended to secondary diols and diesters to afford chiral polyesters. This process is an example of iterative tandem catalysis (ITC), a polymerization method where the concurrent action of two
Fulai Yang et al.
Scientific reports, 9(1), 18067-18067 (2019-12-04)
Camptothecin (CPT), a natural alkaloid isolated from Camptotheca acuminata Decne, is found to show potential insecticidal activities with unique action mechanisms by targeting at DNA-topoisomease I (Top1) complex and inducing cell apoptosis. To improve the efficacy against insect pests, two
Liping Wang et al.
Pesticide biochemistry and physiology, 139, 46-52 (2017-06-10)
Camptothecin (CPT), a quinolone alkaloid extracted from Camptotheca acuminata Decne, exhibits potential insecticidal activities against various insect species. Our previous studies have showed that CPT induced apoptosis in Spodoptera exigua Hübner cell line and inhibited the relaxation activity of topoisomerase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service