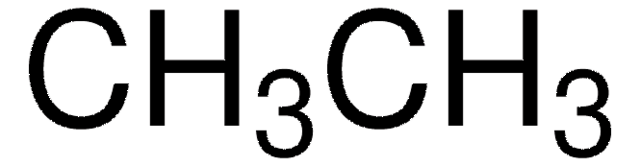About This Item
Recommended Products
vapor density
1.05 (vs air)
Quality Level
vapor pressure
37.95 atm ( 21.1 °C)
Assay
99.99%
form
gas
autoignition temp.
881 °F
expl. lim.
13 %
bp
−88 °C (lit.)
mp
−172 °C (lit.)
density
0.362 g/mL at 20 °C (lit.)
SMILES string
CC
InChI
1S/C2H6/c1-2/h1-2H3
InChI key
OTMSDBZUPAUEDD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- High-pressure oxidation of ethane: The paper discusses the oxidation properties of ethane under high pressure, providing insights crucial for developing combustion models and understanding ethane′s behavior in various industrial processes (H Hashemi, JG Jacobsen, CT Rasmussen, 2017).
- Progress and prospects in catalytic ethane aromatization: This review highlights the advancements in converting ethane to more valuable aromatic hydrocarbons, showcasing the potential of ethane as a petrochemical feedstock (Y Xiang, H Wang, J Cheng, J Matsubu, 2018).
Packaging
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Other Notes
Legal Information
also commonly purchased with this product
control valve
hose barb
purge valve
recommended
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
nwg
Flash Point(F)
-211.0 °F - closed cup
Flash Point(C)
-135 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of Oxygen; Acetylene; Carbon dioxide; Nitrogen; Ethylene; Ethane; Carbon monoxide; Methane
Separation of Hydrogen; Oxygen; Nitrogen; Carbon monoxide; Methane; Carbon dioxide; Acetylene; Ethylene; Ethane
Separation of Methane; Acetylene; Carbon monoxide; Water; Nitrogen; Carbon dioxide; Ethane; Ethylene
Protocol for GC Analysis of C1-C5 Hydrocarbons on Alumina Sulfate PLOT
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service