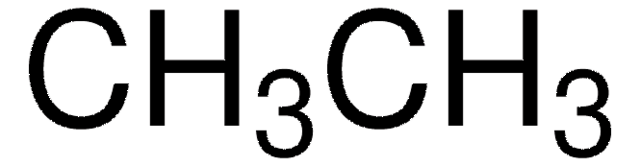About This Item
Recommended Products
vapor density
1.05 (vs air)
Quality Level
vapor pressure
37.95 atm ( 21.1 °C)
Assay
≥99%
form
gas
autoignition temp.
881 °F
expl. lim.
13 %
bp
−88 °C (lit.)
mp
−172 °C (lit.)
density
0.362 g/mL at 20 °C (lit.)
SMILES string
CC
InChI
1S/C2H6/c1-2/h1-2H3
InChI key
OTMSDBZUPAUEDD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Ethylene via oxidative dehydrogenation reaction using different catalytic systems.
- Ethanol via oxidation reaction, which is catalyzed by cytochrome P450 BM3.
- Carbon nanofibers via decomposition method using nickel catalyst supported by a carbon nanotube.
Packaging
Compatible with the following:
- Aldrich® lecture-bottle station systems
- Aldrich® lecture-bottle gas regulators
Other Notes
Legal Information
also commonly purchased with this product
control valve
hose barb
purge valve
recommended
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
nwg
Flash Point(F)
-211.0 °F - closed cup
Flash Point(C)
-135 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Separation of Oxygen; Acetylene; Carbon dioxide; Nitrogen; Ethylene; Ethane; Carbon monoxide; Methane
Separation of Hydrogen; Oxygen; Nitrogen; Carbon monoxide; Methane; Carbon dioxide; Acetylene; Ethylene; Ethane
Separation of Methane; Acetylene; Carbon monoxide; Water; Nitrogen; Carbon dioxide; Ethane; Ethylene
Protocol for GC Analysis of C1-C5 Hydrocarbons on Alumina Sulfate PLOT
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service